Estrogen receptor α regulates the IKKs/NF-kB activity involved in the development of mechanical allodynia induced by REM sleep deprivation in rats
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Several signaling pathways that converge in NF-kB activation have been linked to developing and maintaining different types of pathological pain. In addition, some mechanisms implied in the establishment of chronic pain have been demonstrated to have a sex-dependent correlation. This study aimed to determine if the IKKs/NF-kB signaling pathway is involved in establishing REM sleep deprivation (REMSD) induced mechanical allodynia in rats and its possible regulation depending on estradiol and estrogen receptors. Intrathecal administration of BMS-345541 or minocycline, two drugs that reduce the IKKs/NF-kB activity, avoided the development of mechanical allodynia in female but not in male rats subjected to 48 h of REMSD. Ovariectomy in female rats abolished the effect of BMS-345541 and minocycline. Meanwhile, the 17-β-estradiol restitution restored it. Intrathecal administration of MPP, a selective ERα antagonist, but not PHTPP, a selective ERβ antagonist, avoided the effect of BMS-345541 in female rats without hormonal manipulation. In addition, the transient run-down of ERα in female rats abolished the effect of BMS-345541. All data suggest an important role of ERα as a regulator of the IKKs/NF-kB activity. REMSD increased the ERα protein expression in the dorsal root ganglia and the dorsal spinal cord in females but not in male rats. Interestingly, ERα activation or ERα overexpression allowed the effect of BMS-345541 in male rats. Data suggest an important regulatory role of ERα in the IKKs/NF-kB activity on establishing mechanical allodynia induced by REMSD in female rats.
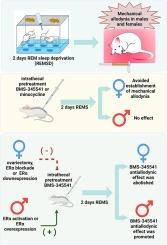
雌激素受体α调节IKKs/NF-kB的活性,IKKs/NF-kB的活性参与了快速眼动睡眠剥夺诱导的大鼠机械异感的发展。
NF-kB 激活所汇聚的几种信号通路与不同类型病理性疼痛的发生和维持有关。此外,慢性疼痛的某些形成机制已被证实与性别相关。本研究旨在确定 IKKs/NF-kB 信号通路是否参与了快速眼动睡眠剥夺(REMSD)诱导的大鼠机械异感的形成,以及雌二醇和雌激素受体对其可能的调节作用。鞘内注射 BMS-345541 或米诺环素(这两种药物可降低 IKKs/NF-kB 的活性)可避免雌性大鼠(而非雄性大鼠)在接受 48 小时 REMSD 后出现机械异感。雌性大鼠卵巢切除术取消了 BMS-345541 和米诺环素的作用。与此同时,17-β-雌二醇的恢复作用则可使其复原。鞘内注射选择性ERα拮抗剂MPP,而非选择性ERβ拮抗剂PHTPP,可避免BMS-345541对雌性大鼠的影响,而无需激素操作。此外,雌性大鼠体内ERα的瞬时下降也会消除BMS-345541的作用。所有数据都表明,ERα 是 IKKs/NF-kB 活性的重要调节因子。REMSD 增加了雌性大鼠背根神经节和脊髓背侧的 ERα 蛋白表达,而雄性大鼠则没有。有趣的是,ERα激活或ERα过表达可使 BMS-345541 对雄性大鼠产生影响。数据表明,ERα 在 IKKs/NF-kB 活动中对雌性大鼠由 REMSD 诱发的机械异动症的建立起了重要的调节作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


