A multifactorial lens on risk factors promoting the progression of Alzheimer’s disease
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
The prevalence of Alzheimer’s disease (AD) among adults has continued to increase over the last two decades, which has sparked a significant increase in research that focuses on the topic of “brain health.” While AD is partially determined by a genetic predisposition, there are still numerous pathophysiological factors that require further research. This research requirement stems from the acknowledgment that AD is a multifactorial disease that to date, cannot be prevented. Therefore, addressing and understanding the potential AD risk factors is necessary to increase the quality of life of an aging population. To raise awareness of critical pathways that impact AD progression, this review manuscript describes AD etiologies, structural impairments, and biomolecular changes that can significantly increase the risk of AD. Among them, a special highlight is given to inflammasomes, which have been shown to bolster neuroinflammation. Alike, the role of brain-derived neurotrophic factor, an essential neuropeptide that promotes the preservation of cognition is presented. In addition, the functional role of neurovascular units to regulate brain health is highlighted and contrasted to inflammatory conditions, such as cellular senescence, vascular damage, and increased visceral adiposity, who all increase the risk of neuroinflammation. Altogether, a multifactorial interventional approach is warranted to reduce the risk of AD.
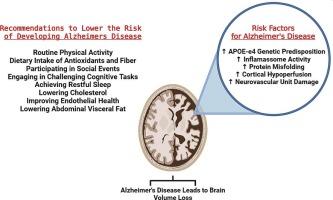
多因素透视促进阿尔茨海默病发展的风险因素。
过去二十年来,阿尔茨海默病(AD)在成年人中的发病率持续上升,这引发了以 "大脑健康 "为主题的研究的大幅增长。虽然阿兹海默症部分是由遗传倾向决定的,但仍有许多病理生理因素需要进一步研究。这一研究要求源于人们认识到,注意力缺失症是一种多因素疾病,至今无法预防。因此,要提高老龄人口的生活质量,就必须解决和了解潜在的注意力缺失症风险因素。为了提高人们对影响注意力缺失症进展的关键途径的认识,本综述手稿介绍了可显著增加注意力缺失症风险的注意力缺失症病因、结构损伤和生物分子变化。其中,炎性体被特别强调,它已被证明能促进神经炎症。此外,还介绍了脑源性神经营养因子的作用,它是一种重要的神经肽,可促进认知能力的保持。此外,还强调了神经血管单元在调节大脑健康方面的功能作用,并将其与细胞衰老、血管损伤和内脏脂肪增加等炎症条件进行了对比,这些因素都会增加神经炎症的风险。总之,需要采取多因素干预方法来降低罹患注意力缺失症的风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


