Discovery of CLKs inhibitors for the treatment of non-small cell lung cancer
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
Targeted inhibition of the Wnt pathway is a promising strategy for treating NSCLC. CDC2-like kinase 2 (CLK2), a dual-specificity kinase responsible for phosphorylating serine/arginine-rich (SR) proteins, can modulate Wnt signaling through the alternative splicing of Wnt target genes, making CLK2 an attractive therapeutic target for NSCLC. In this study, we report the synthesis, optimization, and evaluation of CLK2 inhibitors that effectively suppress the proliferation of NSCLC cells, with the identification of the lead compound LBM22. Notably, compound LBM22 demonstrated potent inhibition of CLK2 (IC50 = 3.9 nM), leading to broad suppression of NSCLC cells proliferation and induction of apoptosis. Furthermore, LBM22 dose-dependently suppressed SR protein phosphorylation (pSRSF4, pSRSF5, and pSRSF6) in NSCLC cells, while downregulating the expression of Wnt pathway-related proteins (p-β-catenin, Axin 2, and c-Myc) as well as anti-apoptotic proteins (Bcl-2 and Mcl-1). Additionally, significant antiproliferative activity was observed for LBM22 in 3D cultured H1975OR cells. In conclusion, LBM22 emerges as a promising CLK2 inhibitor for the treatment of NSCLC.
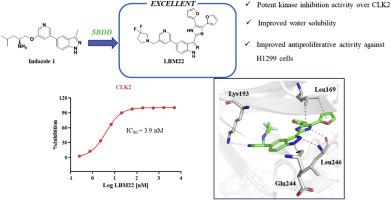
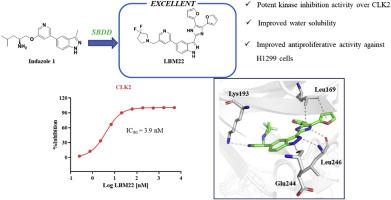
发现治疗非小细胞肺癌的 CLKs 抑制剂
靶向抑制Wnt通路是治疗NSCLC的一种前景广阔的策略。CDC2样激酶2(CLK2)是一种负责磷酸化丝氨酸/富精氨酸(SR)蛋白的双特异性激酶,它可以通过Wnt靶基因的替代剪接来调节Wnt信号转导,从而使CLK2成为治疗NSCLC的一个有吸引力的靶点。在本研究中,我们报告了有效抑制 NSCLC 细胞增殖的 CLK2 抑制剂的合成、优化和评估,并确定了先导化合物 LBM22。值得注意的是,化合物 LBM22 对 CLK2 具有强效抑制作用(IC50 = 3.9 nM),可广泛抑制 NSCLC 细胞增殖并诱导细胞凋亡。此外,LBM22 还能剂量依赖性地抑制 NSCLC 细胞中的 SR 蛋白磷酸化(pSRSF4、pSRSF5 和 pSRSF6),同时下调 Wnt 通路相关蛋白(p-β-catenin、Axin 2 和 c-Myc)以及抗凋亡蛋白(Bcl-2 和 Mcl-1)的表达。此外,在三维培养的 H1975OR 细胞中也观察到了 LBM22 的显著抗增殖活性。总之,LBM22 是一种治疗 NSCLC 很有前景的 CLK2 抑制剂。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


