Oriental covalent immobilization of N-glycan binding protein via N-terminal selective modification
IF 5.7
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Lectin affinity chromatography is one of powerful tools for the study of protein glycosylation. Different lectin proteins can recognize different structures of monosaccharides or oligosaccharide units, allowing for the selective separation of glycopeptides or glycoproteins containing different polysaccharide structures. However, the N-glycans were only partially captured by most of common lectins, reducing the coverage rate of identifying N-glycoconjugates. Recently, it has been reported that the engineering variant of glycan binding protein Fbs1 has a high affinity for innermost Man3GlcNAc2 structure and is able to bind diverse types of N-glycans, which can be suitable for the analysis of protein N-glycosylation. However, efficient immobilization of protein to separation matrix is particularly challenging as it requires the functionality and integrity of the protein to be preserved. Herein, we describe a simple and robust strategy for oriental covalent immobilization of proteins on magnetic nanoparticles by N-terminal selective labeling techniques. We inserted the enterokinase cleavage site to produce the specific N- terminal glycine of protein. Under physiological conditions, the protein was immobilized on the surface of the MNPs by this glycine tag, and the enrichment process could be completed within 30 min. A whole enrichment and purification of glycan and glycopeptides were completed and analyzed by MALDI TOF-MS. The functional materials achieved stable enrichment of glycan structure in different enzyme digestion systems or complex samples, showing excellent anti-interference and applicability.
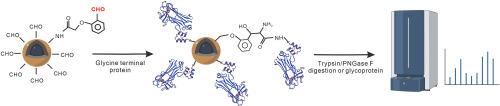
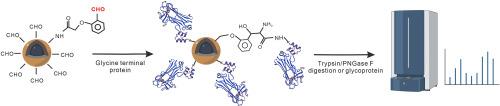
通过 N 端选择性修饰实现 N-聚糖结合蛋白的东方共价固定化
凝集素亲和层析是研究蛋白质糖基化的有力工具之一。不同的凝集素蛋白可以识别不同结构的单糖或寡糖单位,从而选择性地分离含有不同多糖结构的糖肽或糖蛋白。然而,大多数普通凝集素只能捕获部分 N-聚糖,从而降低了鉴定 N-聚糖共轭物的覆盖率。最近有报道称,糖结合蛋白 Fbs1 的工程变体对最内层的 Man3GlcNAc2 结构具有很高的亲和力,能够结合多种类型的 N-聚糖,适用于蛋白质 N-糖基化的分析。然而,将蛋白质高效固定到分离基质上是一项特别具有挑战性的工作,因为这需要保持蛋白质的功能性和完整性。在本文中,我们介绍了一种通过 N 端选择性标记技术将蛋白质共价固定在磁性纳米粒子上的简单而稳健的东方策略。我们插入了肠激酶的裂解位点,以产生特定的蛋白质 N 端甘氨酸。在生理条件下,蛋白质被甘氨酸标签固定在磁性纳米粒子表面,富集过程可在 30 分钟内完成。完成了整个聚糖和聚糖肽的富集和纯化过程,并通过 MALDI TOF-MS 进行了分析。该功能材料在不同的酶解体系或复杂样品中都能实现对聚糖结构的稳定富集,具有良好的抗干扰性和适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Analytica Chimica Acta
化学-分析化学
CiteScore
10.40
自引率
6.50%
发文量
1081
审稿时长
38 days
期刊介绍:
Analytica Chimica Acta has an open access mirror journal Analytica Chimica Acta: X, sharing the same aims and scope, editorial team, submission system and rigorous peer review.
Analytica Chimica Acta provides a forum for the rapid publication of original research, and critical, comprehensive reviews dealing with all aspects of fundamental and applied modern analytical chemistry. The journal welcomes the submission of research papers which report studies concerning the development of new and significant analytical methodologies. In determining the suitability of submitted articles for publication, particular scrutiny will be placed on the degree of novelty and impact of the research and the extent to which it adds to the existing body of knowledge in analytical chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


