MX2 forms nucleoporin-comprising cytoplasmic biomolecular condensates that lure viral capsids
IF 20.6
1区 医学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
Human myxovirus resistance 2 (MX2) can restrict HIV-1 and herpesviruses at a post-entry step through a process requiring an interaction between MX2 and the viral capsids. The involvement of other host cell factors, however, remains poorly understood. Here, we mapped the proximity interactome of MX2, revealing strong enrichment of phenylalanine-glycine (FG)-rich proteins related to the nuclear pore complex as well as proteins that are part of cytoplasmic ribonucleoprotein granules. MX2 interacted with these proteins to form multiprotein cytoplasmic biomolecular condensates that were essential for its anti-HIV-1 and anti-herpes simplex virus 1 (HSV-1) activity. MX2 condensate formation required the disordered N-terminal region and MX2 dimerization. Incoming HIV-1 and HSV-1 capsids associated with MX2 at these dynamic cytoplasmic biomolecular condensates, preventing nuclear entry of their viral genomes. Thus, MX2 forms cytoplasmic condensates that likely act as nuclear pore decoys, trapping capsids and inducing premature viral genome release to interfere with nuclear targeting of HIV-1 and HSV-1.
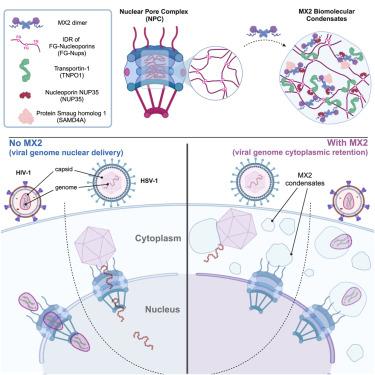
MX2 形成由核蛋白组成的细胞质生物分子凝聚体,引诱病毒外壳
人类肌瘤病毒抵抗力 2(MX2)可通过 MX2 与病毒外壳之间的相互作用,在进入后阶段限制 HIV-1 和疱疹病毒。然而,人们对其他宿主细胞因子的参与仍然知之甚少。在这里,我们绘制了 MX2 的近距离相互作用组,发现与核孔复合体有关的富含苯丙氨酸-甘氨酸(FG)的蛋白质以及作为细胞质核糖核蛋白颗粒一部分的蛋白质大量富集。MX2 与这些蛋白相互作用,形成多蛋白细胞质生物分子凝聚体,这对其抗 HIV-1 和抗单纯疱疹病毒 1(HSV-1)的活性至关重要。MX2 凝聚物的形成需要无序的 N 端区域和 MX2 的二聚化。传入的 HIV-1 和 HSV-1 包囊与 MX2 在这些动态的细胞质生物分子凝聚体上结合,阻止了病毒基因组进入细胞核。因此,MX2 形成的细胞质凝聚体可能充当核孔诱饵,诱捕噬菌体并诱导病毒基因组过早释放,从而干扰 HIV-1 和 HSV-1 的核靶向作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell host & microbe
生物-微生物学
CiteScore
45.10
自引率
1.70%
发文量
201
审稿时长
4-8 weeks
期刊介绍:
Cell Host & Microbe is a scientific journal that was launched in March 2007. The journal aims to provide a platform for scientists to exchange ideas and concepts related to the study of microbes and their interaction with host organisms at a molecular, cellular, and immune level. It publishes novel findings on a wide range of microorganisms including bacteria, fungi, parasites, and viruses. The journal focuses on the interface between the microbe and its host, whether the host is a vertebrate, invertebrate, or plant, and whether the microbe is pathogenic, non-pathogenic, or commensal. The integrated study of microbes and their interactions with each other, their host, and the cellular environment they inhabit is a unifying theme of the journal. The published work in Cell Host & Microbe is expected to be of exceptional significance within its field and also of interest to researchers in other areas. In addition to primary research articles, the journal features expert analysis, commentary, and reviews on current topics of interest in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


