Synthesis of two-dimensional bismuth molybdenum oxide (2D-BMO) nanosheets and their application as fluorescent probes for the detection of explosive nitroaromatic compounds
IF 5.8
2区 环境科学与生态学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
2D-BMO fluorescent nanosheets were synthesized using a solvothermal method followed by probe sonication. These nanosheets were then employed as fluorescent probes for detecting nitroaromatic compounds picric acid (PA) and 2,4-dinitrophenylhydrazine (2,4-DNPH), which have recently garnered significant attention due to their explosive nature and environmental impact. The fluorescent nanoplatform exhibited stable fluorescence emission at a wavelength of 400 nm (λem) when excited at 340 nm (λex). The fluorescence quenching response towards PA and 2,4-DNPH was assessed within a concentration range of 50 to 2000 nM, showing linear responses within the ranges of 50–1100 nM and 50–1400 nM, respectively. The limits of detection were determined to be 2.21 nM for PA and 2.30 nM for 2,4-DNPH, with corresponding R2 values of 0.999 and 0.994. The interaction between the 2D-BMO nanosheets and nitroaromatics was the synergistic combination of the FRET, IFE, and electrostatic interaction, which was studied and explained by UV-visible absorption spectroscopy and zeta potential analysis. This 2D-BMO fluorescent sensor could pave the way for replacing traditional dyes that pose significant harm to the environment in the foreseeable future.
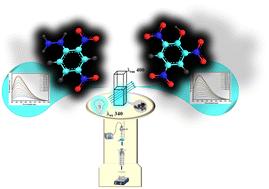
二维氧化钼铋(2D-BMO)纳米片的合成及其作为荧光探针在爆炸性硝基芳香族化合物检测中的应用
采用溶热法合成了二维-BMO 荧光纳米片,然后进行探针超声处理。这些纳米片被用作检测硝基芳香族化合物苦味酸(PA)和 2,4-二硝基苯肼(2,4-DNPH)的荧光探针。该荧光纳米平台在 340 纳米波长(λex)激发时,在 400 纳米波长(λem)处表现出稳定的荧光发射。PA 和 2,4-DNPH 的荧光淬灭响应在 50 至 2000 nM 的浓度范围内进行了评估,分别在 50-1100 nM 和 50-1400 nM 的范围内显示出线性响应。PA 和 2,4-DNPH 的检测限分别为 2.21 nM 和 2.30 nM,相应的 R2 值分别为 0.999 和 0.994。二维-BMO 纳米片与硝基芳香族化合物之间的相互作用是 FRET、IFE 和静电作用的协同组合,紫外可见吸收光谱和 ZETA 电位分析对此进行了研究和解释。在可预见的未来,这种二维-BMO 荧光传感器将为取代对环境造成严重危害的传统染料铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environmental Science: Nano
CHEMISTRY, MULTIDISCIPLINARY-ENVIRONMENTAL SCIENCES
CiteScore
12.20
自引率
5.50%
发文量
290
审稿时长
2.1 months
期刊介绍:
Environmental Science: Nano serves as a comprehensive and high-impact peer-reviewed source of information on the design and demonstration of engineered nanomaterials for environment-based applications. It also covers the interactions between engineered, natural, and incidental nanomaterials with biological and environmental systems. This scope includes, but is not limited to, the following topic areas:
Novel nanomaterial-based applications for water, air, soil, food, and energy sustainability
Nanomaterial interactions with biological systems and nanotoxicology
Environmental fate, reactivity, and transformations of nanoscale materials
Nanoscale processes in the environment
Sustainable nanotechnology including rational nanomaterial design, life cycle assessment, risk/benefit analysis
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


