Jamming of nephron-forming niches in the developing mouse kidney creates cyclical mechanical stresses
IF 37.2
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
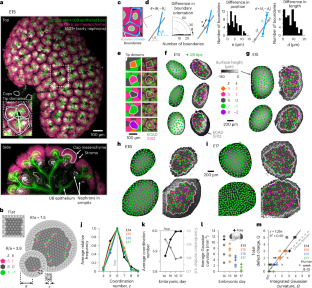
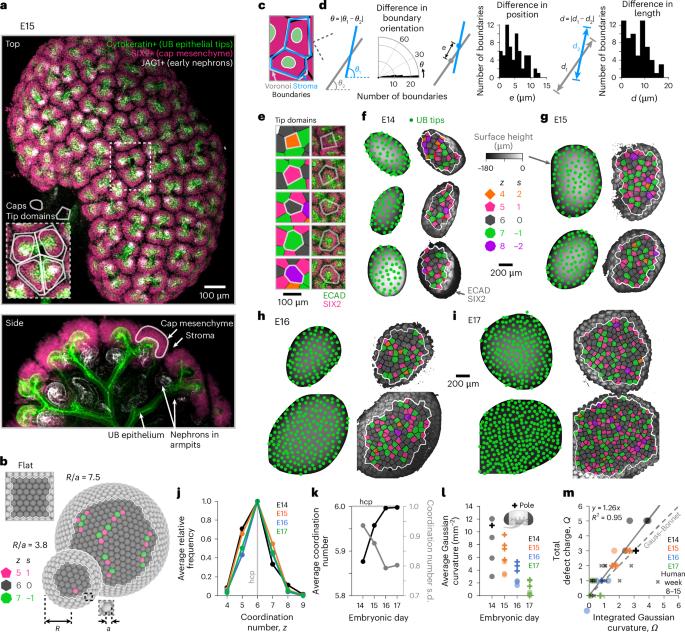
Urinary collecting tubules form during kidney embryogenesis through the branching of the ureteric bud epithelium. A travelling mesenchyme niche of nephron progenitor cells caps each branching ureteric bud tip. These ‘tip domain’ niches pack more closely over developmental time and their number relates to nephron endowment at birth. Yet, how the crowded tissue environment impacts niche number and cell decision-making remains unclear. Here, through experiments and mathematical modelling, we show that niche packing conforms to physical limitations imposed by kidney curvature. We relate packing geometries to rigidity theory to predict a stiffening transition starting at embryonic day 15 in the mouse, validated by micromechanical analysis. Using a method to estimate tip domain ‘ages’ relative to their most recent branch events, we find that new niches overcome mechanical resistance as they branch and displace neighbours. This creates rhythmic mechanical stress in the niche. These findings expand our understanding of kidney development and inform engineering strategies for synthetic regenerative tissues. Geometric packing of tubules in the developing kidney urinary collecting system leads to tissue stiffening and rhythmic mechanical stresses local to nephron-forming niches that synchronize with tubule branching.
发育中的小鼠肾脏中肾小球形成龛位的干扰会产生周期性机械应力
肾脏胚胎发育过程中,输尿管芽上皮细胞分支形成集尿小管。每个输尿管芽的分支顶端都有一个由肾脏祖细胞组成的游动间质龛。随着发育时间的推移,这些 "顶端域 "壁龛会更紧密地聚集在一起,它们的数量与出生时肾小球的禀赋有关。然而,拥挤的组织环境如何影响壁龛数量和细胞决策仍不清楚。在这里,通过实验和数学建模,我们证明了肾小球龛包装符合肾脏曲率所施加的物理限制。我们将包装几何形状与刚度理论联系起来,预测了小鼠胚胎第 15 天开始的僵化转变,并通过微机械分析进行了验证。我们使用一种方法来估算顶端结构域相对于其最近分支事件的 "年龄",结果发现新的壁龛在分支并取代邻近壁龛时会克服机械阻力。这就在龛中产生了有节奏的机械应力。这些发现拓展了我们对肾脏发育的理解,并为合成再生组织的工程策略提供了信息。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Materials
工程技术-材料科学:综合
CiteScore
62.20
自引率
0.70%
发文量
221
审稿时长
3.2 months
期刊介绍:
Nature Materials is a monthly multi-disciplinary journal aimed at bringing together cutting-edge research across the entire spectrum of materials science and engineering. It covers all applied and fundamental aspects of the synthesis/processing, structure/composition, properties, and performance of materials. The journal recognizes that materials research has an increasing impact on classical disciplines such as physics, chemistry, and biology.
Additionally, Nature Materials provides a forum for the development of a common identity among materials scientists and encourages interdisciplinary collaboration. It takes an integrated and balanced approach to all areas of materials research, fostering the exchange of ideas between scientists involved in different disciplines.
Nature Materials is an invaluable resource for scientists in academia and industry who are active in discovering and developing materials and materials-related concepts. It offers engaging and informative papers of exceptional significance and quality, with the aim of influencing the development of society in the future.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


