Ruthenate perovskite with face-sharing motifs for alkaline hydrogen evolution
IF 11.5
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
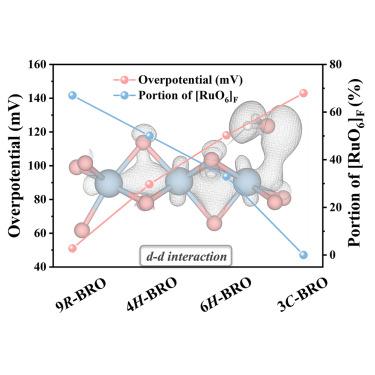
One of the challenges in designing efficient electrocatalysts is rationalizing the impact of crystal and electronic structures on catalytic activity. Here, we synthesized four BaRuO3 polymorphs with different structures and investigated the d-d interaction stemming from face-sharing RuO6. The polymorph 9R-BaRuO3 has the highest percentage of face-sharing RuO6 octahedra and, therefore, the strongest d-d interaction; it shows the best activity and stability in catalyzing alkaline hydrogen evolution reactions (HER). Specifically, 9R-BaRuO3 displays a small Tafel slope of 30 mV dec−1 and a low overpotential of η10 < 51 mV. This performance is attributed to its high intrinsic activity delivered by the d-d interaction and is intimately related to the crystal structure. The micron-sized 9R-BaRuO3 powders are stable under industrial plasma spraying and carry a current density of 0.4 A/cm2 @ 1.74 V in commercial alkaline water electrolyzers. The results on catalytic activities and crystal structure provide insight for designing better electrocatalysts for practical applications.
具有面共享结构的钌酸包晶用于碱性氢进化
设计高效电催化剂所面临的挑战之一是合理解释晶体和电子结构对催化活性的影响。在此,我们合成了四种具有不同结构的 BaRuO3 多晶体,并研究了共面 RuO6 产生的 d-d 相互作用。多晶体 9R-BaRuO3 具有最高比例的共面 RuO6 八面体,因此具有最强的 d-d 相互作用;它在催化碱性氢进化反应(HER)中表现出最佳的活性和稳定性。具体来说,9R-BaRuO3 的塔菲尔斜率较小,为 30 mV dec-1,过电位较低,为 η10 < 51 mV。这种性能归因于其通过 d-d 相互作用产生的高内在活性,并与晶体结构密切相关。微米大小的 9R-BaRuO3 粉末在工业等离子喷涂条件下非常稳定,在商用碱性水电解槽中的电流密度为 0.4 A/cm2 @ 1.74 V。催化活性和晶体结构方面的研究结果为设计更好的电催化剂提供了启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.50
自引率
6.40%
发文量
0
期刊介绍:
Chem Catalysis is a monthly journal that publishes innovative research on fundamental and applied catalysis, providing a platform for researchers across chemistry, chemical engineering, and related fields. It serves as a premier resource for scientists and engineers in academia and industry, covering heterogeneous, homogeneous, and biocatalysis. Emphasizing transformative methods and technologies, the journal aims to advance understanding, introduce novel catalysts, and connect fundamental insights to real-world applications for societal benefit.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


