Modeling Parkinson’s disease pathology in human dopaminergic neurons by sequential exposure to α-synuclein fibrils and proinflammatory cytokines
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
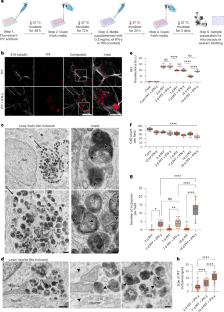
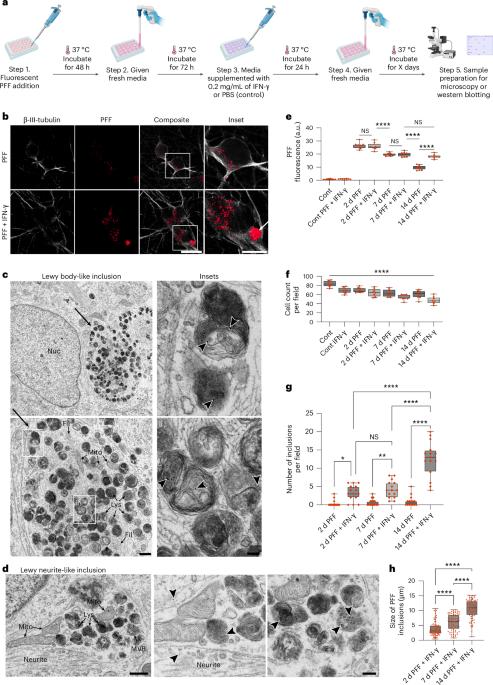
Lewy bodies (LBs), α-synuclein-enriched intracellular inclusions, are a hallmark of Parkinson’s disease (PD) pathology, yet a cellular model for LB formation remains elusive. Recent evidence indicates that immune dysfunction may contribute to the development of PD. In this study, we found that induced pluripotent stem cell (iPSC)-derived human dopaminergic (DA) neurons form LB-like inclusions after treatment with α-synuclein preformed fibrils (PFFs) but only when coupled to a model of immune challenge (interferon-γ or interleukin-1β treatment) or when co-cultured with activated microglia-like cells. Exposure to interferon-γ impairs lysosome function in DA neurons, contributing to LB formation. The knockdown of LAMP2 or the knockout of GBA in conjunction with PFF administration is sufficient for inclusion formation. Finally, we observed that the LB-like inclusions in iPSC-derived DA neurons are membrane bound, suggesting that they are not limited to the cytoplasmic compartment but may be formed due to dysfunctions in autophagy. Together, these data indicate that immune-triggered lysosomal dysfunction may contribute to the development of PD pathology. Bayati et al. discovered that sequential treatment of iPSC-derived dopaminergic neurons with α-synuclein fibrils and proinflammatory cytokines leads to the formation of Lewy body–like inclusions, through the downregulation of lysosomal proteins.
通过连续暴露于α-突触核蛋白纤维和促炎细胞因子,在人类多巴胺能神经元中模拟帕金森病的病理变化
路易体(Lewy bodies,LBs)是富含α-突触核蛋白的细胞内包涵体,是帕金森病(Parkinson's disease,PD)病理学的一个标志,但路易体形成的细胞模型仍未找到。最近的证据表明,免疫功能失调可能会导致帕金森病的发生。在这项研究中,我们发现诱导多能干细胞(iPSC)衍生的人多巴胺能(DA)神经元在接受α-突触核蛋白预成纤维(PFFs)处理后会形成LB样包涵体,但只有在与免疫挑战模型(干扰素-γ或白细胞介素-1β处理)结合或与活化的小胶质细胞类共同培养时才会形成LB样包涵体。暴露于干扰素-γ会损害DA神经元溶酶体的功能,导致LB形成。LAMP2 的敲除或 GBA 的敲除与 PFF 的给药相结合足以导致包涵体的形成。最后,我们观察到,iPSC衍生的DA神经元中的LB样包涵体是膜结合的,这表明它们并不局限于细胞质区,而可能是由于自噬功能障碍而形成的。这些数据共同表明,免疫触发的溶酶体功能障碍可能会导致帕金森病的病理发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


