Multi-omic human pancreatic islet endoplasmic reticulum and cytokine stress response mapping provides type 2 diabetes genetic insights
IF 27.7
1区 生物学
Q1 CELL BIOLOGY
引用次数: 0
Abstract
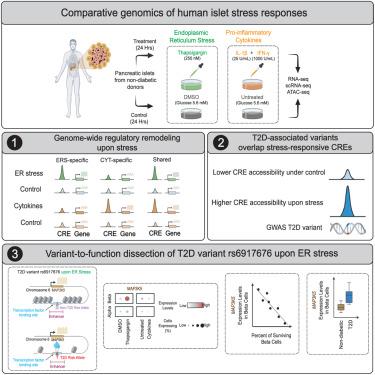
Endoplasmic reticulum (ER) and inflammatory stress responses contribute to islet dysfunction in type 2 diabetes (T2D). Comprehensive genomic understanding of these human islet stress responses and whether T2D-associated genetic variants modulate them is lacking. Here, comparative transcriptome and epigenome analyses of human islets exposed ex vivo to these stressors revealed 30% of expressed genes and 14% of islet cis-regulatory elements (CREs) as stress responsive, modulated largely in an ER- or cytokine-specific fashion. T2D variants overlapped 86 stress-responsive CREs, including 21 induced by ER stress. We linked the rs6917676-T T2D risk allele to increased islet ER-stress-responsive CRE accessibility and allele-specific β cell nuclear factor binding. MAP3K5, the ER-stress-responsive putative rs6917676 T2D effector gene, promoted stress-induced β cell apoptosis. Supporting its pro-diabetogenic role, MAP3K5 expression correlated inversely with human islet β cell abundance and was elevated in T2D β cells. This study provides genome-wide insights into human islet stress responses and context-specific T2D variant effects.
多组学人类胰岛内质网和细胞因子应激反应图谱提供 2 型糖尿病遗传学见解
内质网(ER)和炎症应激反应导致了 2 型糖尿病(T2D)患者的胰岛功能障碍。目前还缺乏对这些人类胰岛应激反应以及 T2D 相关基因变异是否会调节这些反应的全面基因组学了解。在这里,对体内外暴露于这些应激源的人类胰岛进行的转录组和表观基因组比较分析显示,30%的表达基因和14%的胰岛顺式调节元件(CRE)具有应激反应性,主要以ER或细胞因子特异性的方式进行调节。T2D 变异与 86 个应激反应 CRE 重叠,其中 21 个由 ER 应激诱导。我们将 rs6917676-T T2D 风险等位基因与胰岛 ER 应激反应性 CRE 可及性增加和等位基因特异性 β 细胞核因子结合联系起来。MAP3K5是ER应激反应的推定rs6917676 T2D效应基因,可促进应激诱导的β细胞凋亡。MAP3K5的表达与人类胰岛β细胞的丰度成反比,并在T2Dβ细胞中升高,这支持了它的促糖尿病作用。这项研究提供了有关人类胰岛应激反应和特异性 T2D 变异效应的全基因组洞察力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell metabolism
生物-内分泌学与代谢
CiteScore
48.60
自引率
1.40%
发文量
173
审稿时长
2.5 months
期刊介绍:
Cell Metabolism is a top research journal established in 2005 that focuses on publishing original and impactful papers in the field of metabolic research.It covers a wide range of topics including diabetes, obesity, cardiovascular biology, aging and stress responses, circadian biology, and many others.
Cell Metabolism aims to contribute to the advancement of metabolic research by providing a platform for the publication and dissemination of high-quality research and thought-provoking articles.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


