Histone lactylation drives CD8+ T cell metabolism and function
IF 27.7
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
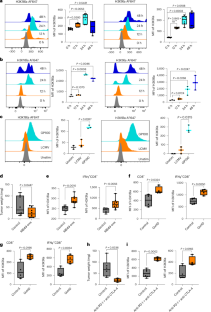
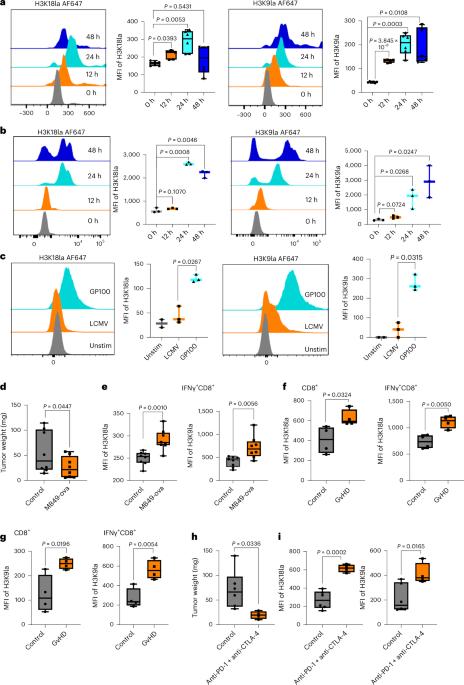
The activation and functional differentiation of CD8+ T cells are linked to metabolic pathways that result in the production of lactate. Lactylation is a lactate-derived histone post-translational modification; however, the relevance of histone lactylation in the context of CD8+ T cell activation and function is not known. Here, we show the enrichment of H3K18 lactylation (H3K18la) and H3K9 lactylation (H3K9la) in human and mouse CD8+ T cells, which act as transcription initiators of key genes regulating CD8+ T cell function. Further, we note distinct patterns of H3K18la and H3K9la in CD8+ T cell subsets linked to their specific metabolic profiles. Additionally, we find that modulation of H3K18la and H3K9la by targeting metabolic and epigenetic pathways influence CD8+ T cell effector function, including antitumor immunity, in preclinical models. Overall, our study uncovers the potential roles of H3K18la and H3K9la in CD8+ T cells. Goswami and colleagues describe how lactylation of histone lysine residues regulates the transcriptome, metabolism and function of CD8+ T cells.
组蛋白乳酰化驱动 CD8+ T 细胞的新陈代谢和功能
CD8+ T细胞的活化和功能分化与产生乳酸的代谢途径有关。乳化是一种乳酸盐衍生的组蛋白翻译后修饰;然而,组蛋白乳化与 CD8+ T 细胞活化和功能的相关性尚不清楚。在这里,我们展示了人类和小鼠 CD8+ T 细胞中 H3K18 乳化(H3K18la)和 H3K9 乳化(H3K9la)的富集,它们是调控 CD8+ T 细胞功能的关键基因的转录启动子。此外,我们注意到 CD8+ T 细胞亚群中 H3K18la 和 H3K9la 的不同模式与其特定的代谢特征有关。此外,我们发现在临床前模型中,通过靶向代谢和表观遗传途径调节 H3K18la 和 H3K9la 会影响 CD8+ T 细胞效应功能,包括抗肿瘤免疫。总之,我们的研究揭示了 H3K18la 和 H3K9la 在 CD8+ T 细胞中的潜在作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Immunology
医学-免疫学
CiteScore
40.00
自引率
2.30%
发文量
248
审稿时长
4-8 weeks
期刊介绍:
Nature Immunology is a monthly journal that publishes the highest quality research in all areas of immunology. The editorial decisions are made by a team of full-time professional editors. The journal prioritizes work that provides translational and/or fundamental insight into the workings of the immune system. It covers a wide range of topics including innate immunity and inflammation, development, immune receptors, signaling and apoptosis, antigen presentation, gene regulation and recombination, cellular and systemic immunity, vaccines, immune tolerance, autoimmunity, tumor immunology, and microbial immunopathology. In addition to publishing significant original research, Nature Immunology also includes comments, News and Views, research highlights, matters arising from readers, and reviews of the literature. The journal serves as a major conduit of top-quality information for the immunology community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


