Tuning a bioengineered hydrogel for studying astrocyte reactivity in glioblastoma
IF 9.6
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
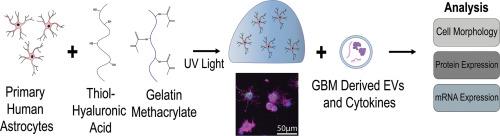
Astrocytes play many essential roles in the central nervous system (CNS) and are altered significantly in disease. These reactive astrocytes contribute to neuroinflammation and disease progression in many pathologies, including glioblastoma (GB), an aggressive form of brain cancer. Current in vitro platforms do not allow for accurate modeling of reactive astrocytes. In this study, we sought to engineer a simple bioengineered hydrogel platform that would support the growth of primary human astrocytes and allow for accurate analysis of various reactive states. After validating this platform using morphological analysis and qPCR, we then used the platform to begin investigating how astrocytes respond to GB derived extracellular vesicles (EVs) and soluble factors (SF). These studies reveal that EVs and SFs induce distinct astrocytic states. In future studies, this platform can be used to study how astrocytes transform the tumor microenvironment in GB and other diseases of the CNS.
Statement of significance
Recent work has shown that astrocytes help maintain brain homeostasis and may contribute to disease progression in diseases such as glioblastoma (GB), a deadly primary brain cancer. In vitro models allow researchers to study basic mechanisms of astrocyte biology in healthy and diseased conditions, however current in vitro systems do not accurately mimic the native brain microenvironment. In this study, we show that our hydrogel system supports primary human astrocyte culture with an accurate phenotype and allows us to study how astrocytes change in response to a variety of inflammatory signals in GB. This platform could be used further investigate astrocyte behavior and possible therapeutics that target reactive astrocytes in GB and other brain diseases.
调整生物工程水凝胶以研究胶质母细胞瘤中星形胶质细胞的反应性
星形胶质细胞在中枢神经系统(CNS)中发挥着许多重要作用,并在疾病中发生显著变化。这些反应性星形胶质细胞在包括胶质母细胞瘤(一种侵袭性脑癌)在内的多种病理情况下都会导致神经炎症和疾病进展。目前的体外平台无法对反应性星形胶质细胞进行精确建模。在这项研究中,我们试图设计一种简单的生物工程水凝胶平台,以支持原代人类星形胶质细胞的生长,并准确分析各种反应状态。通过形态分析和 qPCR 验证该平台后,我们利用该平台开始研究星形胶质细胞如何对国标衍生的细胞外囊泡 (EV) 和可溶性因子 (SF) 作出反应。这些研究表明,EV 和 SF 能诱导不同的星形胶质细胞状态。在未来的研究中,这一平台可用于研究星形胶质细胞如何改变 GB 和中枢神经系统其他疾病的肿瘤微环境。意义说明:最近的研究表明,星形胶质细胞有助于维持大脑的稳态,并可能促进胶质母细胞瘤(GB)等疾病的进展,胶质母细胞瘤是一种致命的原发性脑癌。研究人员可以利用体外模型研究星形胶质细胞在健康和患病情况下的基本生物学机制,但目前的体外系统并不能准确模拟原生大脑微环境。在这项研究中,我们发现我们的水凝胶系统支持原代人类星形胶质细胞培养,并具有准确的表型,使我们能够研究星形胶质细胞如何对 GB 中的各种炎症信号做出反应。该平台可用于进一步研究星形胶质细胞的行为,以及针对 GB 和其他脑部疾病中反应性星形胶质细胞的可能疗法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


