Acoustic resonance technology and quality by design approach facilitate the development of the robust tetrandrine nano-delivery system
IF 4.3
2区 医学
Q1 PHARMACOLOGY & PHARMACY
European Journal of Pharmaceutics and Biopharmaceutics
Pub Date : 2024-10-04
DOI:10.1016/j.ejpb.2024.114522
引用次数: 0
Abstract
The aim of this study was to develop a sufficiently robust tetrandrine (Tet) nano-delivery system using acoustic resonance (AR) technology and freeze-drying technology. This system can effectively improve the solubility and dissolution properties of Tet, along with high stability and scale-up adaptability. Firstly, 54 stabilizers were screened simultaneously in a high-throughput manner with the help of AR technology to fully explore the optimal prescription space of tetrandrine nanosuspension (Tet-NS). The Plackett-Burman design was used to screen for critical variables severely affecting the quality of Tet-NS. The Box-Behnken design was used to investigate and optimize critical variables to obtain optimal nanosuspensions. The optimal prescription was successfully scaled up by 100 times, which was the initial exploration of its commercial scale production. Solidification studies have shown that formulations with 2.44% fructose as the cryoprotectant have excellent redispersibility. Compared with pure Tet, Tet in Tet-NS showed a significant increase in solubility and dissolution rate in water. Fourier transform infrared (FT-IR) demonstrated that no significant interactions occurred between the drug and excipients in Tet-NS. Powder x-ray diffraction analysis (PXRD) indicated that some of the Tet transformed into amorphous state during the preparation process. In short-term stability study, Tet-NS successfully maintained its physical stability. In summary, under the guidance of the QbD concept, this study rapidly developed Tet-NS using acoustic resonance technology, which can effectively improve the solubility and dissolution properties of Tet. During the development of Tet-NS, AR technology has demonstrated high particle size reduction capability, the ability to process multiple sets of formulations in parallel, and excellent scale-up capability. Meanwhile, the method and concept of this study are not limited to Tet, but also applicable to other poorly water-soluble drugs.
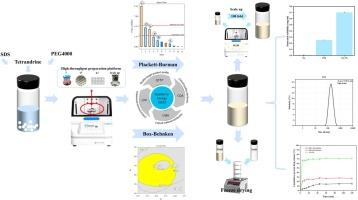
声共振技术和质量设计方法促进了坚固耐用的四氢化萘纳米给药系统的开发。
本研究的目的是利用声共振(AR)技术和冷冻干燥技术开发一种足够稳健的四氢化萘(Tet)纳米给药系统。该系统能有效提高 Tet 的溶解度和溶解性能,同时具有高稳定性和放大适应性。首先,利用声共振技术高通量地同时筛选了54种稳定剂,以充分探索四氢化萘磺酸纳米悬浮剂(Tet-NS)的最佳处方空间。采用褶皱-伯曼设计筛选严重影响 Tet-NS 质量的关键变量。盒-贝肯设计用于研究和优化关键变量,以获得最佳纳米悬浮剂。最佳处方成功放大了 100 倍,这是对其商业规模生产的初步探索。凝固研究表明,以 2.44% 的果糖作为低温保护剂的配方具有极佳的再分散性。与纯 Tet 相比,Tet-NS 中的 Tet 在水中的溶解度和溶解速率显著提高。傅立叶变换红外光谱(FT-IR)显示,Tet-NS 中的药物和辅料之间没有发生明显的相互作用。粉末 X 射线衍射分析(PXRD)表明,部分 Tet 在制备过程中转变为无定形状态。在短期稳定性研究中,Tet-NS 成功地保持了其物理稳定性。综上所述,本研究在 QbD 理念的指导下,利用声共振技术快速开发了 Tet-NS,有效改善了 Tet 的溶解性和溶出性能。在 Tet-NS 的开发过程中,AR 技术表现出了较高的粒径减小能力、并行处理多组制剂的能力以及出色的放大能力。同时,本研究的方法和理念不仅限于 Tet,也适用于其他水溶性较差的药物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
8.80
自引率
4.10%
发文量
211
审稿时长
36 days
期刊介绍:
The European Journal of Pharmaceutics and Biopharmaceutics provides a medium for the publication of novel, innovative and hypothesis-driven research from the areas of Pharmaceutics and Biopharmaceutics.
Topics covered include for example:
Design and development of drug delivery systems for pharmaceuticals and biopharmaceuticals (small molecules, proteins, nucleic acids)
Aspects of manufacturing process design
Biomedical aspects of drug product design
Strategies and formulations for controlled drug transport across biological barriers
Physicochemical aspects of drug product development
Novel excipients for drug product design
Drug delivery and controlled release systems for systemic and local applications
Nanomaterials for therapeutic and diagnostic purposes
Advanced therapy medicinal products
Medical devices supporting a distinct pharmacological effect.
文献相关原料
公司名称
产品信息
索莱宝
Polyethylene glycol 400 (PEG 400)
索莱宝
Polyethylene glycol 4000 (PEG 4000)
索莱宝
Polyethylene glycol 6000 (PEG 6000)
上海源叶
Polyvinyl alcohol (PVA)
上海源叶
Chitosan
上海源叶
Sodium dodecyl sulfate (SDS)
上海源叶
Tween 80
上海源叶
Span 60
阿拉丁
Hydroxypropyl cellulose (HPC)

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


