Resveratrol inhibits white adipose deposition by the ESR1-mediated PI3K/AKT signaling pathway
IF 4.4
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Excessive adipose accumulation is the primary cause of obesity. Resveratrol (RES), a natural polyphenolic compound, has garnered significant attention for its anti-obesity properties. However, the precise mechanisms by which RES influences fat deposition have not yet been explored. In this study, the aim was to identify the target proteins and associated pathways of RES in order to elucidate the mechanisms by which RES reduces fat deposition. In this study, mice were administered 400 mg/kg of RES via gavage for 12 weeks. We found that while 400 mg/kg RES had no impact on the growth of the mice, it significantly reduced the weight of various white adipose tissues, as well as the serum and liver concentrations of total cholesterol and triglycerides. Network pharmacology identified 15 potential targets of RES and highlighted the PI3K/AKT signaling pathway as a key pathway. Molecular docking and dynamic simulations suggested that ESR1 might be the target protein through which RES exerts its anti-fat deposition effects. In vitro experiments revealed that ESR1 promotes the proliferation and inhibits the differentiation of 3 T3-L1 adipocytes, and suppresses the PI3K/AKT signaling pathway. Silencing the ESR1 gene altered the ability of RES to inhibit cell differentiation via the PI3K/AKT pathway. Gene expression results in subcutaneous adipose tissue, epididymal fat tissue, and liver tissue of mice were consistent with observations in cells. In summary, RES reduces white fat deposition by directly targeting the ESR1 protein and inhibiting the PI3K/AKT signaling pathway. Our findings provide new insights into the potential use of RES in the prevention and treatment of obesity.
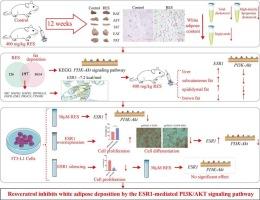
白藜芦醇通过 ESR1 介导的 PI3K/AKT 信号通路抑制白色脂肪沉积。
脂肪过度堆积是肥胖的主要原因。白藜芦醇(RES)是一种天然多酚化合物,因其抗肥胖特性而备受关注。然而,RES 影响脂肪沉积的确切机制尚未探明。本研究旨在确定 RES 的靶蛋白和相关途径,以阐明 RES 减少脂肪沉积的机制。在这项研究中,通过灌胃给小鼠服用每公斤 400 毫克的 RES,持续 12 周。我们发现,虽然每公斤 400 毫克的 RES 对小鼠的生长没有影响,但它能显著降低各种白色脂肪组织的重量,以及血清和肝脏中总胆固醇和甘油三酯的浓度。网络药理学确定了 RES 的 15 个潜在靶点,并强调 PI3K/AKT 信号通路是一个关键通路。分子对接和动态模拟表明,ESR1可能是RES发挥抗脂肪沉积作用的靶蛋白。体外实验发现,ESR1能促进3个T3-L1脂肪细胞的增殖和抑制分化,并抑制PI3K/AKT信号通路。沉默 ESR1 基因改变了 RES 通过 PI3K/AKT 途径抑制细胞分化的能力。小鼠皮下脂肪组织、附睾脂肪组织和肝组织中的基因表达结果与细胞中的观察结果一致。总之,RES通过直接靶向ESR1蛋白和抑制PI3K/AKT信号通路来减少白色脂肪沉积。我们的研究结果为RES在预防和治疗肥胖症方面的潜在应用提供了新的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cellular signalling
生物-细胞生物学
CiteScore
8.40
自引率
0.00%
发文量
250
审稿时长
27 days
期刊介绍:
Cellular Signalling publishes original research describing fundamental and clinical findings on the mechanisms, actions and structural components of cellular signalling systems in vitro and in vivo.
Cellular Signalling aims at full length research papers defining signalling systems ranging from microorganisms to cells, tissues and higher organisms.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


