A Triple-Mismatch Differentiating assay exploiting activation and trans cleavage of CRISPR-Cas12a for mutation detection with ultra specificity and sensitivity
IF 10.7
1区 生物学
Q1 BIOPHYSICS
引用次数: 0
Abstract
Liquid biopsy technology is non-invasive and convenient, and is currently an emerging technology for cancer screening. Among them, clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR associated protein 12a (Cas12a) based nucleic acid detection technology has the advantages of high sensitivity, rapidity, and easy operation. However, CRISPR-Cas12a does not discriminate single-base mismatches of targets well enough to meet the needs of clinical detection. Herein, we developed the Triple-Mismatch Differentiating (TMD) assay. This assay amplified the small thermodynamic difference in mismatches at one site at the level of CRISPR-Cas12a activation to a significant thermodynamic difference at three sites at both the level of CRISPR-Cas12a activation and trans-cleavage, which greatly improves the ability of CRISPR-Cas12a to discriminate between base mismatches. Our manipulation greatly improved the specificity of the CRISPR-Cas12a system while maintaining its inherent sensitivity and simplicity, increasing the detection limit to 0.0001%. When testing samples from pancreatic cancer patients, our results were highly consistent with NGS sequencing results. We believe that the TMD assay will provide a new technology for early cancer detection and will be widely used in the clinical practice.
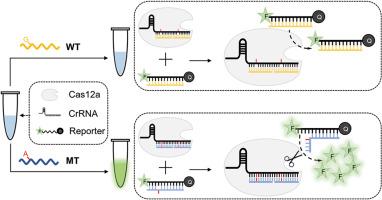
利用 CRISPR-Cas12a 的激活和反式裂解进行突变检测的三重错配分化测定,具有超特异性和灵敏度。
液体活检技术具有无创、便捷的特点,是目前新兴的癌症筛查技术。其中,基于簇状规则间隔短回文重复序列(CRISPR)-CRISPR相关蛋白12a(Cas12a)的核酸检测技术具有灵敏度高、快速、操作简便等优点。然而,CRISPR-Cas12a不能很好地分辨目标物的单碱基错配,无法满足临床检测的需要。在此,我们开发了三重错配分辨(TMD)检测方法。这种检测方法将CRISPR-Cas12a活化水平上一个位点的微小错配热力学差异放大到CRISPR-Cas12a活化和反式裂解水平上三个位点的显著热力学差异,从而大大提高了CRISPR-Cas12a对碱基错配的分辨能力。我们的操作大大提高了CRISPR-Cas12a系统的特异性,同时保持了其固有的灵敏度和简单性,将检测限提高到了0.0001%。在检测胰腺癌患者样本时,我们的结果与 NGS 测序结果高度一致。我们相信,TMD 检测将为早期癌症检测提供一项新技术,并将广泛应用于临床实践。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biosensors and Bioelectronics
工程技术-电化学
CiteScore
20.80
自引率
7.10%
发文量
1006
审稿时长
29 days
期刊介绍:
Biosensors & Bioelectronics, along with its open access companion journal Biosensors & Bioelectronics: X, is the leading international publication in the field of biosensors and bioelectronics. It covers research, design, development, and application of biosensors, which are analytical devices incorporating biological materials with physicochemical transducers. These devices, including sensors, DNA chips, electronic noses, and lab-on-a-chip, produce digital signals proportional to specific analytes. Examples include immunosensors and enzyme-based biosensors, applied in various fields such as medicine, environmental monitoring, and food industry. The journal also focuses on molecular and supramolecular structures for enhancing device performance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


