NIR-Actuated Ferroptosis Nanomotor for Enhanced Tumor Penetration and Therapy
IF 27.4
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Ferroptosis nano-inducers have drawn considerable attention in the treatment of malignant tumors. However, low intratumoral hydrogen peroxide level and complex biological barriers hinder the ability of nanomedicines to generate sufficient reactive oxygen species (ROS) and achieve tumor penetration. Here a near-infrared (NIR)-driven ROS self-supplying nanomotor is successfully designed for synergistic tumor chemodynamic therapy (CDT) and photothermal therapy (PTT). Janus nanomotor is created by the asymmetrical modification of polydopamine (PDA) with zinc peroxide (ZnO2) and subsequent ferrous ion (Fe2+) chelation via the polyphenol groups from the PDA, here refer as ZnO2@PDA-Fe (Z@P-F). ZnO2 is capable of slowly releasing hydrogen peroxide (H2O2) into an acidic tumor microenvironment (TME) providing sufficient ingredients for the Fenton reaction necessary for ferroptosis. Upon NIR laser irradiation, the loaded Fe2+ is released and a thermal gradient is simultaneously formed owing to the asymmetric PDA coating, thus endowing the nanomotor with self-thermophoresis based enhanced diffusion for subsequent lysosomal escape and tumor penetration. Therefore, the release of ferrous ions (Fe2+), self-supplied H2O2, and self-thermophoresis of nanomotors with NIR actuation further improve the synergistic CDT/PTT efficacy, showing great potential for active tumor therapy.
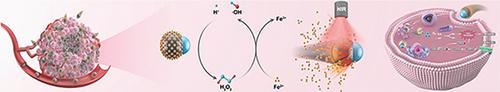
用于增强肿瘤穿透和治疗的近红外激活铁氧体纳米马达
纳米铁诱导剂在治疗恶性肿瘤方面备受关注。然而,瘤内过氧化氢水平低和复杂的生物屏障阻碍了纳米药物产生足够的活性氧(ROS)和实现肿瘤穿透的能力。本文成功设计了一种近红外(NIR)驱动的ROS自供纳米马达,用于协同肿瘤化学动力疗法(CDT)和光热疗法(PTT)。Janus纳米马达是通过过氧化锌(ZnO2)对聚多巴胺(PDA)进行不对称修饰,然后通过PDA中的多酚基团螯合亚铁离子(Fe2+)而产生的,这里称为ZnO2@PDA-Fe(Z@P-F)。ZnO2 能够在酸性肿瘤微环境(TME)中缓慢释放过氧化氢(H2O2),为铁跃迁所需的芬顿反应提供足够的成分。在近红外激光照射下,负载的 Fe2+ 被释放,同时由于不对称的 PDA 涂层而形成热梯度,从而使纳米马达具有基于自热泳的增强扩散能力,以便随后逃逸溶酶体并穿透肿瘤。因此,亚铁离子(Fe2+)的释放、自供H2O2和纳米马达的自热泳与近红外驱动进一步提高了CDT/PTT的协同疗效,显示出主动治疗肿瘤的巨大潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Materials
工程技术-材料科学:综合
CiteScore
43.00
自引率
4.10%
发文量
2182
审稿时长
2 months
期刊介绍:
Advanced Materials, one of the world's most prestigious journals and the foundation of the Advanced portfolio, is the home of choice for best-in-class materials science for more than 30 years. Following this fast-growing and interdisciplinary field, we are considering and publishing the most important discoveries on any and all materials from materials scientists, chemists, physicists, engineers as well as health and life scientists and bringing you the latest results and trends in modern materials-related research every week.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


