Degradation Mechanism and Enhanced Stability of Organolithium for Chemical Lithiation
IF 24.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Organolithium solutions, especially Li-arene solutions (LASs) with high reactivity and controllable redox potentials, have gained significant attention because of their wide applications in chemical lithiation, liquid anodes, and battery recycling. However, the sudden loss of reactivity when stored or applied at room temperature is still puzzling and inhibits the application of LASs. In this work, the degradation mechanism of LASs is fully investigated and revealed by combining various experimental characterization and computational simulations. A hierarchical reaction mechanism for lithium biphenyl/2-methyl tetrahydrofuran (Li-Bp-2MT), a lithiation solution used for most anodes, explains degradation and side product formation. Specifically, the dimerization of the active component Li1Bp[2MT]1 forms an inactive dimer that is irreversibly reduced in the presence of locally accumulated highly reductive Li0. This reaction mechanism reveals the atomic origins of lithiation solution deactivation and accounts for all solid and gaseous byproducts. LiH is identified as the dominating solid byproduct, indicating irreversible destruction of the active components and facilitating side reactions producing H2 and CH4. Based on reaction mechanism insights, modifying molecular interactions and reaction kinetics are experimentally shown to inhibit Li0 aggregation kinetics, enhancing long-term prelithiation performance. This research provides comprehensive guidelines for practical applications of LASs.
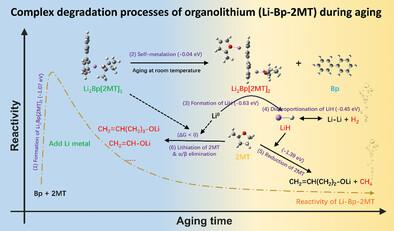
用于化学锂化的有机锂的降解机制和稳定性增强
有机锂溶液,尤其是具有高反应活性和可控氧化还原电位的锂烯溶液(LASs),因其在化学锂化、液体阳极和电池回收方面的广泛应用而备受关注。然而,LASs 在室温下储存或应用时会突然失去活性,这一点仍然令人费解,并阻碍了其应用。本研究结合各种实验表征和计算模拟,全面研究并揭示了锂离子电池的降解机理。联苯/2-甲基四氢呋喃锂(Li-Bp-2MT)是一种用于大多数阳极的锂化溶液,其分层反应机制解释了降解和副产物的形成。具体来说,活性成分 Li1Bp[2MT]1 的二聚体形成了一种非活性二聚体,这种二聚体在局部积累的高还原性 Li0 的存在下被不可逆地还原。这种反应机理揭示了锂化溶液失活的原子起源,并解释了所有固体和气体副产物。LiH 被确定为主要的固体副产物,表明活性成分遭到了不可逆的破坏,并促进了产生 H2 和 CH4 的副反应。基于对反应机理的深入了解,实验证明,改变分子相互作用和反应动力学可抑制 Li0 的聚集动力学,从而提高长期预锂化性能。这项研究为 LAS 的实际应用提供了全面的指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


