A Comprehensive Study on the Parameters Affecting Magnesium Plating/Stripping Kinetics in Rechargeable Mg Batteries
IF 24.4
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
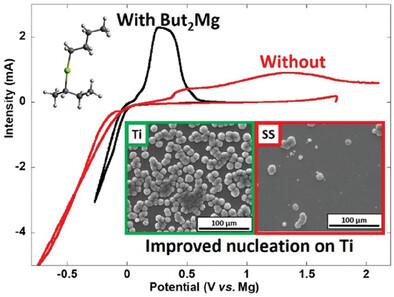
Mg metal anode-based battery is a more sustainable, lower cost, and higher energy density alternative to Li-ion. However, this battery chemistry also faces several challenges associated with the high charge density of Mg2+, including achieving high reversibility and low voltage hysteresis for Mg metal plating/stripping. While significant improvements are achieved in last decades, they involve rather complex electrolyte formulations and/or Mg salts difficult to produce, and the use of unpractical substrates such as platinum. Here, significant improvement in terms of Mg plating kinetics is achieved in electrolytes containing commercial magnesium bis(trifluoromethanesulfonyl)imide salt (Mg(TFSI)2) by using titanium substrate with similar crystal structure and lattice parameter as Mg leading to lower nucleation overpotential. Low salt concentration electrolyte and addition of dibutyl magnesium (Mg(butyl)2) also enable the formation of thinner interphase, richer in solvent based decomposition products, further improving Mg plating kinetics. This work highlights the complex role of Mg(butyl)2, often considered as a simple drying agent, and how it impacts ion solvation favoring the mobility of electroactive cationic species, paving the way toward better electrolyte design with improved cation transference number.
可充电镁电池中影响镀镁/剥离动力学参数的综合研究
镁金属阳极电池是锂离子电池的一种可持续性更强、成本更低、能量密度更高的替代品。然而,这种电池化学也面临着与 Mg2+ 的高电荷密度相关的一些挑战,包括实现金属镁电镀/剥离的高可逆性和低电压滞后。虽然在过去几十年中取得了重大改进,但这些改进涉及相当复杂的电解质配方和/或难以生产的镁盐,以及使用铂等不实用的基质。在这里,通过使用晶体结构和晶格参数与镁相似的钛基底,降低成核过电位,在含有商用双(三氟甲烷磺酰)亚胺镁盐(Mg(TFSI)2)的电解液中实现了镁电镀动力学的重大改进。低盐浓度电解液和二丁基镁(Mg(butyl)2)的添加也能形成更薄的中间相,富含溶剂型分解产物,进一步改善镁电镀动力学。这项研究强调了通常被视为简单干燥剂的 Mg(丁基)2 的复杂作用,以及它如何影响离子溶解,促进电活性阳离子物种的流动性,从而为设计具有更好阳离子转移数量的电解质铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Advanced Energy Materials
CHEMISTRY, PHYSICAL-ENERGY & FUELS
CiteScore
41.90
自引率
4.00%
发文量
889
审稿时长
1.4 months
期刊介绍:
Established in 2011, Advanced Energy Materials is an international, interdisciplinary, English-language journal that focuses on materials used in energy harvesting, conversion, and storage. It is regarded as a top-quality journal alongside Advanced Materials, Advanced Functional Materials, and Small.
With a 2022 Impact Factor of 27.8, Advanced Energy Materials is considered a prime source for the best energy-related research. The journal covers a wide range of topics in energy-related research, including organic and inorganic photovoltaics, batteries and supercapacitors, fuel cells, hydrogen generation and storage, thermoelectrics, water splitting and photocatalysis, solar fuels and thermosolar power, magnetocalorics, and piezoelectronics.
The readership of Advanced Energy Materials includes materials scientists, chemists, physicists, and engineers in both academia and industry. The journal is indexed in various databases and collections, such as Advanced Technologies & Aerospace Database, FIZ Karlsruhe, INSPEC (IET), Science Citation Index Expanded, Technology Collection, and Web of Science, among others.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


