Every reaction Detail Matters: An in silico driven Step-by-Step Guide to understand the B2O3-Catalyzed CO2 to cyclic carbonates conversion
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
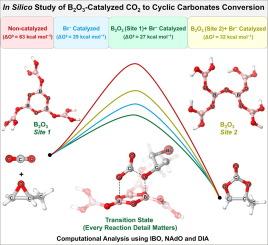
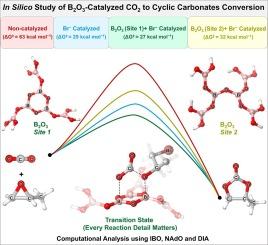
The catalytic conversion of carbon dioxide (CO2) to cyclic organic carbonates (COCs) via cycloaddition with epoxides offers a dual benefit of reducing CO2 emissions while producing valuable chemical products. In this detailed in silico study, we employ density functional theory (DFT) to meticulously investigate the mechanisms underlying the B2O3/n-NBu4Br-catalyzed cycloaddition of CO2 and epoxides, following a step-by-step approach according to the reaction details. We provide a comprehensive comparison of the non-catalyzed, n-NBu4Br alone, and B2O3/n-NBu4Br-catalyzed pathways, emphasizing two distinct active sites within the B2O3 framework: Site 1 (six-membered boroxol ring) and Site 2 (open chain configuration). Our computational analysis highlights that the Site 1-catalyzed reaction pathway is more favorable, featuring a lower overall energy barrier of 26.8 kcal/mol, compared to 32.5 kcal/mol for the Site 2-catalyzed pathway. Detailed intrinsic bond orbital (IBO), natural adaptive orbital (NAdO) and distortion-interaction analysis (DIA) reveal significant differences in bonding properties and energy interactions, elucidating why Site 1 exhibits superior catalytic activity over Site 2. These findings are corroborated by experimental observations, which indicate that ball milling B2O3 increases defect sites, thereby enhancing exposure of active sites Site 1 and improving catalytic performance. This study provides an in-depth understanding of how the intrinsic properties of boron active sites influence the catalytic efficiency of B2O3, offering valuable insights for the design and optimization of metal-free catalysts for CO2 conversion.
每个反应细节都很重要:了解 B2O3 催化二氧化碳到环状碳酸盐转化的硅驱动分步指南
通过环氧化物环加成催化二氧化碳 (CO2) 转化为环状有机碳酸盐 (COC),既能减少二氧化碳排放,又能生产有价值的化学产品,可谓一举两得。在这项详细的硅学研究中,我们采用密度泛函理论(DFT)细致研究了 B2O3/n-NBu4Br 催化的 CO2 与环氧化物环加成反应的机理,并根据反应细节采用了循序渐进的方法。我们对非催化、单独 n-NBu4Br 和 B2O3/n-NBu4Br 催化途径进行了全面比较,强调了 B2O3 框架内两个不同的活性位点:位点 1(六元硼氧环)和位点 2(开链构型)。我们的计算分析表明,位点 1 催化的反应途径更为有利,其总能障较低,仅为 26.8 kcal/mol,而位点 2 催化的反应途径则为 32.5 kcal/mol。详细的固有键轨道(IBO)、自然适应轨道(NAdO)和畸变-相互作用分析(DIA)揭示了成键性质和能量相互作用的显著差异,从而阐明了为什么位点 1 的催化活性优于位点 2。这些发现得到了实验观察的证实,实验观察表明,球磨 B2O3 增加了缺陷位点,从而增加了活性位点 1 的暴露,提高了催化性能。这项研究深入揭示了硼活性位点的内在特性如何影响 B2O3 的催化效率,为设计和优化用于二氧化碳转化的无金属催化剂提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


