Investigating the inhibition of xanthine oxidase by five catechins: Kinetic studies, spectroscopy, molecular docking, and dynamics simulations
IF 7.7
1区 化学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
International Journal of Biological Macromolecules
Pub Date : 2024-10-03
DOI:10.1016/j.ijbiomac.2024.136231
引用次数: 0
Abstract
Catechins compounds from tea have demonstrated significant inhibitory effects on xanthine oxidase (XOD). However, the precise inhibitory mechanisms of the main catechins on XOD remain to be fully elucidated. This study explored the inhibition mechanisms and binding characteristics of five catechins (GC, EGC, EC, EGCG, and ECG) on XOD through a combination of inhibition kinetics, multi-spectroscopy analysis, molecular docking, and dynamics simulations. Among the catechins, EGCG and ECG exhibited the most potent inhibitory activities against XOD. All five catechins were found to exhibit mixed inhibition, affecting the hydrophobic groups and secondary structure of XOD predominantly through hydrophobic interactions and hydrogen bonding. Molecular dynamics simulations revealed that a 3,4,5-trihydroxybenzoic acid moiety at C3 position significantly enhances the binding affinity of EGCG and ECG to XOD. Additionally, the decrease of β-sheet and random coil induced by EGCG and ECG was found to be crucial for enhancing inhibitory activity of XOD. In vitro cell experiments showed that EGCG and ECG significantly reduced high uric acid levels of BRL-3A cell. This study elucidates the inhibitory mechanisms of catechins on XOD, paving the way for their application as XOD inhibitors to combat hyperuricemia.
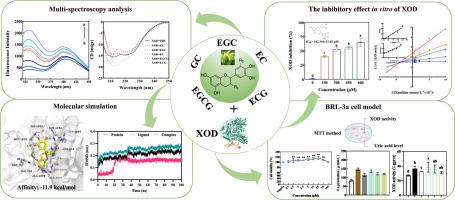
研究五种儿茶素对黄嘌呤氧化酶的抑制作用:动力学研究、光谱学、分子对接和动力学模拟。
茶叶中的儿茶素化合物对黄嘌呤氧化酶(XOD)有明显的抑制作用。然而,主要儿茶素对黄嘌呤氧化酶的确切抑制机制仍有待全面阐明。本研究通过抑制动力学、多光谱分析、分子对接和动力学模拟等方法,探讨了五种儿茶素(GC、EGC、EC、EGCG和ECG)对XOD的抑制机制和结合特性。在儿茶素中,EGCG 和 ECG 对 XOD 的抑制活性最强。研究发现,所有五种儿茶素都表现出混合抑制作用,主要通过疏水相互作用和氢键影响 XOD 的疏水基团和二级结构。分子动力学模拟显示,C3 位的 3,4,5- 三羟基苯甲酸分子能显著增强 EGCG 和 ECG 与 XOD 的结合亲和力。此外,研究还发现,EGCG 和 ECG 诱导的 β-片和无规线圈的减少是增强 XOD 抑制活性的关键。体外细胞实验表明,EGCG 和 ECG 能显著降低 BRL-3A 细胞的高尿酸水平。这项研究阐明了儿茶素对 XOD 的抑制机制,为儿茶素作为 XOD 抑制剂应用于防治高尿酸血症铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
13.70
自引率
9.80%
发文量
2728
审稿时长
64 days
期刊介绍:
The International Journal of Biological Macromolecules is a well-established international journal dedicated to research on the chemical and biological aspects of natural macromolecules. Focusing on proteins, macromolecular carbohydrates, glycoproteins, proteoglycans, lignins, biological poly-acids, and nucleic acids, the journal presents the latest findings in molecular structure, properties, biological activities, interactions, modifications, and functional properties. Papers must offer new and novel insights, encompassing related model systems, structural conformational studies, theoretical developments, and analytical techniques. Each paper is required to primarily focus on at least one named biological macromolecule, reflected in the title, abstract, and text.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


