Measurement of absolute abundance of crystallins in human and αA N101D transgenic mouse lenses using 15N-labeled crystallin standards
IF 3
2区 医学
Q1 OPHTHALMOLOGY
引用次数: 0
Abstract
Stable isotope labeled standards of all major human lens crystallins were created to measure the abundance of lens endogenous crystallins from birth to adulthood. All major human crystallins (αA, αB, βA2, βA3/A1, βA4, βB1, βB2, βB3, γA, γB, γC, γD, γS) were cloned with N-terminal 6 x His tagged SUMO for ease of purification and the ability to generate natural N-termini by SUMO protease cleavage when producing crystallins for structure/function studies. They were then expressed in 15N-enriched media, quantified by mass spectrometry, and mixed in proportions found in young human lens to act as an artificial lens standard. The absolute quantification method was tested using soluble protein from 5-day, 23-day, 18-month, and 18-year-old human lenses spiked with the 15N artificial lens standard. Proteins were trypsinized, relative ratios of light and heavy labeled peptides determined using high-resolution precursor and data independent MS2 scans, and data analysis performed using Skyline software. Crystallin abundances were measured in both human donor lenses and in transgenic mouse αA N101D cataract lenses. Technical replicates of human crystallin abundance measurements were performed with average coefficients of variation of approximately 2% across all 13 crystallins. αA crystallin comprised 27% of the soluble protein of 5-day-old lens and decreased to 16% by 18-years of age. Over this time period αB increased from 6% to 9% and the αA/αB ratio decreased from 4.5/1 to 2/1. γS-crystallin also increased nearly 2-fold from 7% to 12%, becoming the 3rd most abundant protein in adult lens, while βB1 increased from 14% to 20%, becoming the most abundant crystallin of adult lens. Minor crystallins βA2, βB3, and γA comprised only about 1% each of the newborn lens soluble protein, and their abundance dropped precipitously by adulthood. While 9 of the SUMO tagged crystallins were useful for purification of crystallins for structural studies, γA, γB, γC, and γD were resistant to cleavage by SUMO protease. The abundance of WT and N101D human αA in transgenic mouse lenses was approximately 40-fold lower than endogenous mouse αA, but the deamidation mimic human αA N101D was less soluble than human WT αA. The high content of αA and the transient abundance of βA2, βB3, and γA in young lens suggest these crystallins play a role in early lens development and growth. βB1 becoming the most abundant crystallin may result from its role in promoting higher order β-crystallin oligomerization in mature lens. The full set of human crystallin expression vectors in the Addgene repository should be a useful resource for future crystallin studies. 15N labeling of these crystallins will be useful to accurately quantify crystallins in lens anatomic regions, as well as measure the composition of insoluble light scattering crystallin aggregates. The standards will also be useful to measure the abundance of crystallins expressed in transgenic animal models.
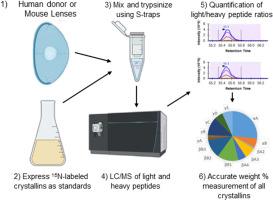
使用 15N 标记的晶体蛋白标准品测量人类和 αA N101D 转基因小鼠晶状体中晶体蛋白的绝对丰度。
建立了所有主要人类晶状体晶状体蛋白的稳定同位素标记标准,以测量从出生到成年期间晶状体内源性晶状体蛋白的丰度。克隆了所有主要人类晶状体蛋白(αA、αB、βA2、βA3/A1、βA4、βB1、βB2、βB3、γA、γB、γC、γD、γS)的 N 端 6 x His 标记 SUMO,以便于纯化,并在生产晶状体蛋白进行结构/功能研究时,能够通过 SUMO 蛋白酶裂解生成天然 N 端。然后,在富含 15N 的培养基中表达它们,用质谱法进行定量,并与人类年轻晶状体中的比例混合,作为人工晶状体标准。使用从 5 天、23 天、18 个月和 18 岁人类晶状体中掺入 15N 人工晶状体标准的可溶性蛋白质,对绝对定量方法进行了测试。对蛋白质进行胰蛋白酶化,使用高分辨率前体和独立数据 MS2 扫描确定轻标肽和重标肽的相对比率,并使用 Skyline 软件进行数据分析。对人类供体晶状体和转基因小鼠 αA N101D 白内障晶状体中的晶体蛋白丰度进行了测定。对人类晶状体蛋白丰度的测量进行了技术重复,所有 13 种晶状体蛋白的平均变异系数约为 2%。αA 晶状体蛋白在 5 天大的晶状体可溶性蛋白中占 27%,到 18 岁时降至 16%。在此期间,αB 从 6% 增加到 9%,αA/αB 的比率从 4.5/1 下降到 2/1。γS 结晶蛋白也从 7% 增加到 12%,增加了近 2 倍,成为成人晶状体中含量第三高的蛋白质,而 βB1 则从 14% 增加到 20%,成为成人晶状体中含量最高的结晶蛋白。次要晶状体βA2、βB3和γA分别只占新生晶状体可溶性蛋白的1%左右,成年后其含量急剧下降。虽然 9 种 SUMO 标记的晶体蛋白有助于纯化晶体蛋白以进行结构研究,但γA、γB、γC 和γD 对 SUMO 蛋白酶的裂解具有抗性。转基因小鼠晶状体中 WT 和 N101D 人 αA 的丰度比内源性小鼠 αA 低约 40 倍,但去氨基化模拟人 αA N101D 的可溶性低于人 WT αA。αA的高含量以及βA2、βB3和γA在幼年晶状体中的短暂丰度表明,这些晶状体蛋白在晶状体的早期发育和生长中发挥作用。βB1之所以成为含量最高的晶状体蛋白,可能是因为它在成熟晶状体中促进高阶β-晶状体蛋白寡聚化的作用。Addgene 资源库中的全套人类晶体蛋白表达载体应该是未来晶体蛋白研究的有用资源。对这些晶体蛋白进行 15N 标记将有助于准确量化晶状体解剖区域中的晶体蛋白,以及测量不溶性光散射晶体蛋白聚集体的组成。这些标准物质还有助于测量转基因动物模型中表达的晶体蛋白的丰度。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental eye research
医学-眼科学
CiteScore
6.80
自引率
5.90%
发文量
323
审稿时长
66 days
期刊介绍:
The primary goal of Experimental Eye Research is to publish original research papers on all aspects of experimental biology of the eye and ocular tissues that seek to define the mechanisms of normal function and/or disease. Studies of ocular tissues that encompass the disciplines of cell biology, developmental biology, genetics, molecular biology, physiology, biochemistry, biophysics, immunology or microbiology are most welcomed. Manuscripts that are purely clinical or in a surgical area of ophthalmology are not appropriate for submission to Experimental Eye Research and if received will be returned without review.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


