PEG-based co-solvent aqueous electrolytes enabling high-energy rechargeable aqueous magnesium-ion batteries
IF 16.8
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
In contrast to organic electrolyte systems, rechargeable aqueous magnesium-ion batteries (RAMIBs) offer unparalleled advantages, particularly in environmental sustainability, safety, cost-effectiveness, and their potential for large-scale energy storage. However, the practical implementation of RAMIBs has been hindered by the limited electrochemical stability of aqueous electrolytes. Although polyethylene glycol (PEG)-based aqueous electrolytes provide a wide electrochemical window, the poor ionic conductivity has restricted their widespread adoption. In this study, we address this challenge by employing trimethyl phosphate (TMP) and PEG400 as the co-solvent of aqueous magnesium-ion electrolytes. This combination significantly enhances the ionic conductivity to 4.44 mS cm-1 and increases the electrochemical stability window of the aqueous electrolyte by mitigating the activity of H2O. In addition, we underscore the role of TMP as a potent co-solvent in modulating the solvation structures of Mg2+ ions and modifying the electrochemical behavior of the electrolytes. Leveraging this advancement, in conjunction with a V2O5 cathode and a 3,4,9,10-perylenetetracarboxylic diimide (PTCDI) anode, the resulting RAMIBs demonstrate a remarkable discharge capacity of 227.6 mAh g-1, a large energy density of 157 Wh kg-1 and a high power density of 70 W kg-1. Our findings represent a significant stride toward exploiting the full potential of RAMIBs for future energy storage applications.
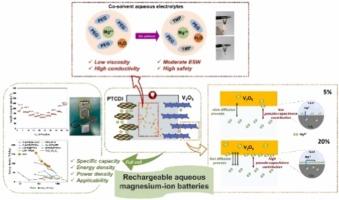
基于 PEG 的辅助溶剂水性电解质可实现高能量可充电镁离子水电池
与有机电解质系统相比,可充电镁离子水电池(RAMIBs)具有无与伦比的优势,尤其是在环境可持续性、安全性、成本效益以及大规模储能的潜力方面。然而,水性电解质的电化学稳定性有限,阻碍了 RAMIB 的实际应用。虽然基于聚乙二醇(PEG)的水基电解质具有较宽的电化学窗口,但离子导电性差限制了其广泛应用。在本研究中,我们采用磷酸三甲酯 (TMP) 和 PEG400 作为镁离子水基电解质的辅助溶剂,以应对这一挑战。这种组合大大提高了离子电导率,使其达到 4.44 mS cm-1,并通过减轻 H2O 的活性提高了水基电解质的电化学稳定性窗口。此外,我们还强调了 TMP 作为一种强效助溶剂在调节 Mg2+ 离子溶解结构和改变电解质电化学行为方面的作用。利用这一先进技术,结合 V2O5 阴极和 3,4,9,10-perylenetetracarboxylic diimide (PTCDI) 阳极,所制备的 RAMIB 显示出 227.6 mAh g-1 的显著放电容量、157 Wh kg-1 的高能量密度和 70 W kg-1 的高功率密度。我们的研究结果标志着在充分挖掘 RAMIB 在未来储能应用中的潜力方面取得了重大进展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


