A DFT and microkinetic modeling study of pressure effects on electroreduction reduction of CO2 to ethanol
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
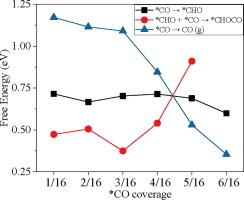
The CO2 electroreduction reaction (CO2RR) is a promising way to convert surplus renewable electricity into valuable low-carbon fuel. Ethanol is one of the most beneficial products because of its high energy density, ready transport, and extensive use. The CO2 partial pressure (PCO2) significantly impacts ethanol formation. This work explores the mechanism of pressure dependence in ethanol formation on the Cu2O/Cu(1 1 1) surface via the density functional theory (DFT) and microkinetic model calculations. DFT results show that *CO is the major adsorbate on the catalyst surface, and its coverage is the descriptor of pressure. Initially, increasing the coverage of *CO promotes the C–C coupling to ethanol, while the excess coverage shifts the potential determining step (PDS) from the *CHO formation to the *CHO–*CO addition, prejudicing the ethanol formation. The degree of selectivity control (DSC) results show that *CO stability positively affects ethanol production, while the stabilities of *H and *CH3CH2OH exhibit the reverse. Their effects decrease and increase with pressure increase, respectively, leading to the volcano plot of the pressure dependence on ethanol formation. The mechanistic insights gained from this work provide relevant guidelines to optimize the reaction pressure conditions for the CO2RR to ethanol reaction.
二氧化碳电还原成乙醇的压力效应 DFT 和微动力学模型研究
二氧化碳电还原反应(CO2RR)是将剩余的可再生电力转化为有价值的低碳燃料的一种可行方法。乙醇是最有益的产品之一,因为它能量密度高、运输方便、用途广泛。二氧化碳分压(PCO2)对乙醇的形成有很大影响。这项研究通过密度泛函理论(DFT)和微动力学模型计算,探索了 Cu2O/Cu(1 1 1) 表面乙醇形成的压力依赖机制。DFT 结果表明,*CO 是催化剂表面的主要吸附剂,其覆盖率是压力的描述因子。起初,增加 *CO 的覆盖率会促进 C-C 偶联生成乙醇,而过量的覆盖率则会将潜在决定步骤(PDS)从 *CHO 生成转移到 *CHO-*CO 加成,从而影响乙醇的生成。选择性控制(DSC)结果表明,*CO 的稳定性对乙醇的生成有积极影响,而 *H 和 *CH3CH2OH 的稳定性则相反。它们的影响分别随着压力的增加而减小和增大,从而形成了乙醇形成的压力依赖性火山图。这项工作所获得的机理启示为优化 CO2RR 转化乙醇反应的压力条件提供了相关指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


