Cerium-regulated Mg3FeO4@biochar activated persulfate to enhance degradation of aqueous tetracycline: The dominant role of cerium
IF 6.3
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The role of cerium (Ce) in Ce-regulated Mg3FeO4@biochar (CMF@BC) and the mechanism by which CMF@BC activates persulfate (PS) remain unclear. In this work, a novel CMF@BC was synthesized through the simple impregnation–pyrolysis process and utilized as a PS activator for the removal of aqueous tetracycline (TC). Under the optimal degradation conditions, the removal efficiency and mineralization rate of TC reached 92.7 % and 84.2 % within 60 min, respectively, the corresponding rate constant was 0.0487 min−1. The CMF@BC catalyst exhibited extremely high stability and excellent reusability after five cycles. The characterization results identified Fe2+ and oxygen vacancies as major catalytic sites. The Ce dopant inhibited the aggregation of metal ions of Mg3FeO4 during the high-temperature pyrolysis process and the Ce3+/Ce4+ redox cycle promoted Fe2+ regeneration, thus improving the catalyst performance. The degradation process was dominated by non-free radical (1O2) pathways. The possible TC-removal pathway was inferred from liquid chromatography–mass spectrometry results and density functional theory calculations. The toxicity of the degradation products was also evaluated. The CMF@BC catalyst displayed excellent catalytic performance and recyclability, wide pH adaptability, and a low ion-leaching rate, confirming its broad application prospects as a PS activator in antibiotic wastewater treatment.
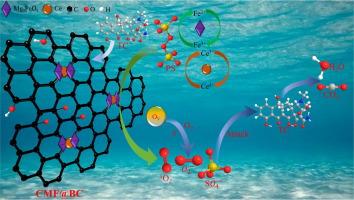
铈调节 Mg3FeO4@biochar 活化过硫酸盐,提高四环素水溶液的降解:铈的主导作用
铈(Ce)在铈调控Mg3FeO4@生物炭(CMF@BC)中的作用以及CMF@BC激活过硫酸盐(PS)的机制仍不清楚。本研究通过简单的浸渍-热解工艺合成了一种新型的 CMF@BC,并将其用作去除水体中四环素(TC)的过硫酸盐活化剂。在最佳降解条件下,60 min内四环素的去除率和矿化率分别达到92.7%和84.2%,相应的速率常数为0.0487 min-1。CMF@BC 催化剂在五个循环后表现出极高的稳定性和良好的重复利用率。表征结果表明,Fe2+ 和氧空位是主要的催化位点。在高温热解过程中,Ce 掺杂抑制了 Mg3FeO4 金属离子的聚集,Ce3+/Ce4+ 氧化还原循环促进了 Fe2+ 的再生,从而提高了催化剂的性能。降解过程以非自由基(1O2)途径为主。根据液相色谱-质谱分析结果和密度泛函理论计算推断出了可能的 TC 清除途径。此外,还对降解产物的毒性进行了评估。CMF@BC催化剂表现出优异的催化性能和可回收性、广泛的pH适应性和较低的离子浸出率,证实了其作为PS活化剂在抗生素废水处理中的广阔应用前景。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Applied Surface Science
工程技术-材料科学:膜
CiteScore
12.50
自引率
7.50%
发文量
3393
审稿时长
67 days
期刊介绍:
Applied Surface Science covers topics contributing to a better understanding of surfaces, interfaces, nanostructures and their applications. The journal is concerned with scientific research on the atomic and molecular level of material properties determined with specific surface analytical techniques and/or computational methods, as well as the processing of such structures.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


