The Role of CH4 in Plasma-Assisted CO2 and CH4 Conversion in a Rotating Gliding Arc Plasma: Insights Revealed by Experiments and Modeling
IF 7.3
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
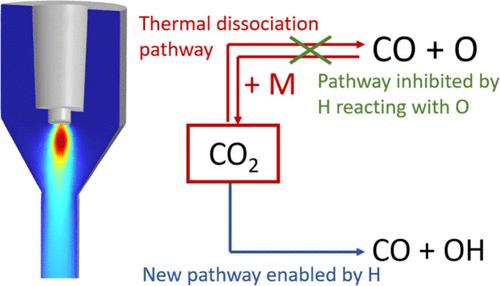
We investigated the combined conversion of CO2 and CH4, so-called dry reforming of methane (DRM), in a rotating gliding arc (RGA) reactor by experiments and modeling for different CO2/CH4 mixing ratios. We obtained the best results at the lowest flow rate (4 L/min) and the lowest amount of CH4 in the feed gas mixture (25%), reaching a conversion of 22% and 39% for CO2 and CH4, respectively, an energy efficiency of 62% and energy cost of 3.25 eV/molecule. A lower energy cost of 2.65 eV/molecule was obtained at 8 L/min. By means of a 3D computational fluid dynamics model, we show that the addition of CH4 reduces the gas temperature inside the plasma, resulting in slower chemical kinetics, explaining why the least amount of CH4 (i.e., 25/75 CH4/CO2) yields the highest CO2 and CH4 conversion. Additionally, the 25/75 CH4/CO2 mixture also displays the highest energy efficiency, due to the high conversion, as well as due the high CO concentration produced in this gas mixture, which is the most beneficial product in terms of energy efficiency. Finally, by means of a quasi-1D chemical kinetics model, we demonstrate that the addition of CH4 suppresses the CO recombination reactions back into CO2, after the plasma, as H-based radicals from CH4 quickly react with O radicals that would otherwise recombine with CO.
等离子体辅助旋转滑弧等离子体中二氧化碳和甲烷转化过程中甲烷的作用:实验和建模揭示的启示
我们通过实验和建模研究了在旋转滑弧式 (RGA) 反应器中对不同 CO2/CH4 混合比的 CO2 和 CH4(即甲烷干转化 (DRM))的联合转化。我们在流速最低(4 升/分钟)、原料气混合物中 CH4 含量最低(25%)的情况下取得了最佳结果,CO2 和 CH4 的转化率分别达到 22% 和 39%,能效达到 62%,能量成本为 3.25 eV/分子。在 8 升/分钟的条件下,能量成本更低,为 2.65 eV/分子。通过三维计算流体动力学模型,我们发现 CH4 的加入会降低等离子体内的气体温度,从而导致化学动力学变慢,这也解释了为什么 CH4 的用量最少(即 25/75 CH4/CO2)却能产生最高的 CO2 和 CH4 转化率。此外,25/75 CH4/CO2 混合物的能效也最高,这不仅是因为转化率高,还因为该混合气体中产生的 CO 浓度高,而 CO 是能效最高的产物。最后,通过一个准一维化学动力学模型,我们证明了在等离子体之后,CH4 的加入会抑制 CO 重合反应,使其回到 CO2 中,因为 CH4 中的 H 基自由基会迅速与 O 基自由基发生反应,否则 O 基自由基会与 CO 重合。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Sustainable Chemistry & Engineering
CHEMISTRY, MULTIDISCIPLINARY-ENGINEERING, CHEMICAL
CiteScore
13.80
自引率
4.80%
发文量
1470
审稿时长
1.7 months
期刊介绍:
ACS Sustainable Chemistry & Engineering is a prestigious weekly peer-reviewed scientific journal published by the American Chemical Society. Dedicated to advancing the principles of green chemistry and green engineering, it covers a wide array of research topics including green chemistry, green engineering, biomass, alternative energy, and life cycle assessment.
The journal welcomes submissions in various formats, including Letters, Articles, Features, and Perspectives (Reviews), that address the challenges of sustainability in the chemical enterprise and contribute to the advancement of sustainable practices. Join us in shaping the future of sustainable chemistry and engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


