Comparative genomics unravels a rich set of biosynthetic gene clusters with distinct evolutionary trajectories across fungal species (Termitomyces) farmed by termites
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
Abstract
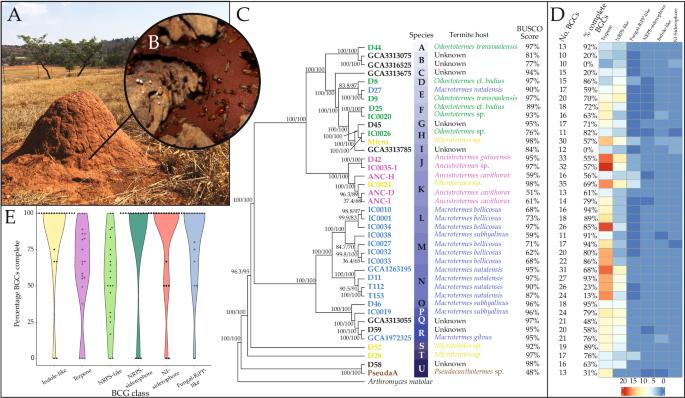
The use of compounds produced by hosts or symbionts for defence against antagonists has been identified in many organisms, including in fungus-farming termites (Macrotermitinae). The obligate mutualistic fungus Termitomyces plays a pivotal role in plant biomass decomposition and as the primary food source for these termites. Despite the isolation of various specialized metabolites from different Termitomyces species, our grasp of their natural product repertoire remains incomplete. To address this knowledge gap, we conducted a comprehensive analysis of 39 Termitomyces genomes, representing 21 species associated with members of five termite host genera. We identified 754 biosynthetic gene clusters (BGCs) coding for specialized metabolites and categorized 660 BGCs into 61 biosynthetic gene cluster families (GCFs) spanning five compound classes. Seven GCFs were shared by all 21 Termitomyces species and 21 GCFs were present in all genomes of subsets of species. Evolutionary constraint analyses on the 25 most abundant GCFs revealed distinctive evolutionary histories, signifying that millions of years of termite-fungus symbiosis have influenced diverse biosynthetic pathways. This study unveils a wealth of non-random and largely undiscovered chemical potential within Termitomyces and contributes to our understanding of the intricate evolutionary trajectories of biosynthetic gene clusters in the context of long-standing symbiosis. Systematic identification and comparison of biosynthetic gene clusters among Termitomyces species provides insight into their evolutionary history and potential functions of BGCs and the chemicals synthesized by these pathways.
比较基因组学揭示了白蚁养殖的真菌物种(白蚁真菌)中具有不同进化轨迹的丰富的生物合成基因簇
在许多生物中都发现了利用宿主或共生体产生的化合物来抵御拮抗剂的现象,其中包括以真菌为生的白蚁(大白蚁科)。必须共生的真菌鸡枞菌在植物生物质分解过程中发挥着关键作用,也是这些白蚁的主要食物来源。尽管从不同的白蚁属真菌中分离出了各种特殊的代谢产物,但我们对其天然产物谱系的了解仍然不全面。为了填补这一知识空白,我们对与五个白蚁宿主属成员相关的 21 个种的 39 个鸡枞菌基因组进行了全面分析。我们确定了 754 个生物合成基因簇(BGCs),这些基因簇编码专门的代谢物,并将 660 个生物合成基因簇分为 61 个生物合成基因簇家族(GCFs),涵盖 5 个化合物类别。所有 21 个鸡枞菌物种共有 7 个 GCF,21 个 GCF 存在于所有物种子集的基因组中。对 25 种最丰富的 GCFs 进行的进化约束分析揭示了其独特的进化历史,表明数百万年的白蚁-真菌共生影响了不同的生物合成途径。这项研究揭示了白蚁真菌中大量非随机的、大部分未被发现的化学潜力,有助于我们了解生物合成基因簇在长期共生过程中错综复杂的进化轨迹。通过系统鉴定和比较鸡枞菌物种间的生物合成基因簇,可以深入了解它们的进化历史、BGCs的潜在功能以及这些途径合成的化学物质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


