Metabolic regulation of mitochondrial morphologies in pancreatic beta cells: coupling of bioenergetics and mitochondrial dynamics
IF 5.2
1区 生物学
Q1 BIOLOGY
引用次数: 0
Abstract
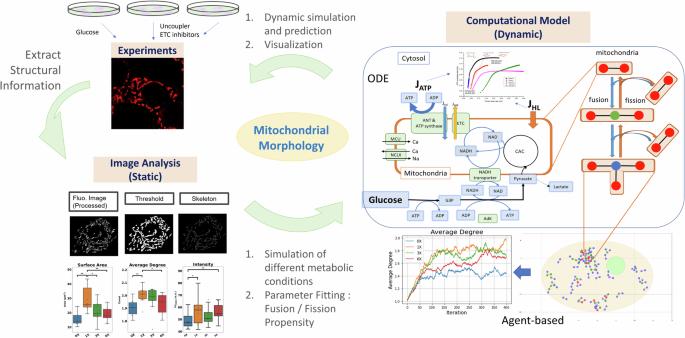
Cellular bioenergetics and mitochondrial dynamics are crucial for the secretion of insulin by pancreatic beta cells in response to elevated levels of blood glucose. To elucidate the interactions between energy production and mitochondrial fission/fusion dynamics, we combine live-cell mitochondria imaging with biophysical-based modeling and graph-based network analysis. The aim is to determine the mechanism that regulates mitochondrial morphology and balances metabolic demands in pancreatic beta cells. A minimalistic differential equation-based model for beta cells is constructed that includes glycolysis, oxidative phosphorylation, calcium dynamics, and fission/fusion dynamics, with ATP synthase flux and proton leak flux as main regulators of mitochondrial dynamics. The model shows that mitochondrial fission occurs in response to hyperglycemia, starvation, ATP synthase inhibition, uncoupling, and diabetic conditions, in which the rate of proton leakage exceeds the rate of mitochondrial ATP synthesis. Under these metabolic challenges, the propensities of tip-to-tip fusion events simulated from the microscopy images of the mitochondrial networks are lower than those in the control group and prevent the formation of mitochondrial networks. The study provides a quantitative framework that couples bioenergetic regulation with mitochondrial dynamics, offering insights into how mitochondria adapt to metabolic challenges. A study combined experiments, image analysis and mathematical modeling to understand the coupling between bioenergetics and mitochondrial dynamics and investigates how excess nutrients and starvation affect mitochondrial morphology.
胰腺β细胞线粒体形态的代谢调节:生物能和线粒体动力学的耦合
细胞生物能和线粒体动力学对于胰腺β细胞在血糖水平升高时分泌胰岛素至关重要。为了阐明能量产生和线粒体裂变/融合动力学之间的相互作用,我们将活细胞线粒体成像与基于生物物理的建模和基于图的网络分析相结合。目的是确定调节线粒体形态和平衡胰岛β细胞代谢需求的机制。该模型包括糖酵解、氧化磷酸化、钙动力学和裂变/融合动力学,其中 ATP 合酶通量和质子漏通量是线粒体动力学的主要调节因子。该模型显示,线粒体在高血糖、饥饿、ATP 合成酶抑制、解偶联和糖尿病条件下会发生裂变,在这些条件下,质子泄漏的速度超过了线粒体 ATP 合成的速度。在这些代谢挑战下,从线粒体网络显微镜图像中模拟出的尖端到尖端融合事件的倾向性低于对照组,从而阻止了线粒体网络的形成。这项研究提供了一个将生物能调节与线粒体动力学结合起来的定量框架,有助于深入了解线粒体如何适应代谢挑战。一项研究结合了实验、图像分析和数学建模,以了解生物能与线粒体动力学之间的耦合,并研究过量营养物质和饥饿如何影响线粒体形态。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Biology
Medicine-Medicine (miscellaneous)
CiteScore
8.60
自引率
1.70%
发文量
1233
审稿时长
13 weeks
期刊介绍:
Communications Biology is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the biological sciences. Research papers published by the journal represent significant advances bringing new biological insight to a specialized area of research.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


