The LINC00319 binding to STAT3 promotes the cell proliferation, migration, invasion and EMT process in oral squamous cell carcinoma
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Background
Long non-coding RNA LINC00319 has been implicated in the progression of various cancers, including oral squamous cell carcinoma (OSCC). While our previous work has revealed some aspects of LINC00319's role in OSCC, including its upregulation and involvement in a competing endogenous RNA (ceRNA) mechanism, the full extent of its functions and regulatory mechanisms in OSCC progression remain to be fully elucidated.
Objective
This study aimed to investigate the function of LINC00319 in OSCC and its potential interaction with the STAT3 signaling pathway, thus uncovering novel regulatory mechanisms and therapeutic targets.
Methods
Bioinformatics analysis was performed using TCGA data to evaluate LINC00319 expression in OSCC tissues and its correlation with STAT3 signaling. The direct binding between LINC00319 and STAT3 was examined by RNA pull-down, FISH, and RIP assays. Functional experiments, including CCK-8, transwell migration and invasion assays, and western blot analysis of EMT markers and STAT3 pathway activation, were conducted to assess the effects of LINC00319 on OSCC cell behaviors and its interaction with the STAT3 signaling pathway. In vivo xenograft models were established to validate the role of LINC00319 in tumor growth and STAT3 activation.
Results
LINC00319 expression was significantly upregulated in OSCC tissues compared to normal tissues, and high LINC00319 expression correlated with STAT3 signaling activation. Mechanistically, LINC00319 directly bound to STAT3 protein and promoted its phosphorylation at Tyr705. LINC00319 overexpression enhanced, while its knockdown suppressed, the proliferation, migration, invasion, and EMT of OSCC cells. These oncogenic effects were mediated through STAT3 activation and could be reversed by the STAT3 inhibitor stattic. In vivo experiments further confirmed that LINC00319 silencing inhibited tumor growth and STAT3 phosphorylation.
Conclusion
This study uncovers that LINC00319 promotes OSCC tumorigenesis by directly binding to and activating STAT3 signaling. These findings provide new insights into the regulatory mechanisms of STAT3 by long non-coding RNAs and highlight the potential of LINC00319 as a biomarker and therapeutic target in OSCC.
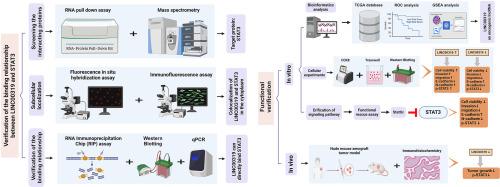
LINC00319 与 STAT3 结合可促进口腔鳞状细胞癌的细胞增殖、迁移、侵袭和 EMT 过程。
背景:长非编码RNA LINC00319与包括口腔鳞状细胞癌(OSCC)在内的多种癌症的进展有关。虽然我们之前的工作已经揭示了LINC00319在OSCC中的某些作用,包括其上调和参与竞争性内源性RNA(ceRNA)机制,但其在OSCC进展中的全部功能和调控机制仍有待全面阐明:本研究旨在探讨LINC00319在OSCC中的功能及其与STAT3信号通路的潜在相互作用,从而发现新的调控机制和治疗靶点:方法:利用TCGA数据进行生物信息学分析,评估LINC00319在OSCC组织中的表达及其与STAT3信号通路的相关性。通过 RNA pull-down、FISH 和 RIP 试验检测了 LINC00319 与 STAT3 的直接结合。为了评估LINC00319对OSCC细胞行为的影响及其与STAT3信号通路的相互作用,研究人员进行了功能实验,包括CCK-8、transwell迁移和侵袭实验,以及EMT标记物和STAT3通路激活的Western印迹分析。建立了体内异种移植模型,以验证LINC00319在肿瘤生长和STAT3激活中的作用:结果:与正常组织相比,LINC00319在OSCC组织中的表达明显上调,LINC00319的高表达与STAT3信号激活相关。从机理上讲,LINC00319直接与STAT3蛋白结合并促进其在Tyr705处磷酸化。LINC00319 的过表达增强了 OSCC 细胞的增殖、迁移、侵袭和 EMT,而其敲除则抑制了这些作用。这些致癌效应是通过 STAT3 激活介导的,并能被 STAT3 抑制剂 stattic 逆转。体内实验进一步证实,沉默LINC00319可抑制肿瘤生长和STAT3磷酸化:本研究发现,LINC00319通过直接与STAT3信号结合并激活STAT3信号来促进OSCC肿瘤发生。这些发现为了解长非编码RNA对STAT3的调控机制提供了新的视角,并凸显了LINC00319作为OSCC生物标志物和治疗靶点的潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


