Harnessing high potential benzothiazole chalcones against dengue virus NS5 protein: A multi-faceted theoretical study through molecular docking, ADME, and DFT
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Chalcones bearing tetralone, indanone and benzothiazole cores were synthesized successfully using a general Claisen-Schmidt condensation protocol. The prepared compounds were purified and structurally analyzed by 1H, 13C NMR, and FT-IR techniques. A multi-faceted theoretical approach, combining Density Functional Theory (DFT), molecular docking, and ADME predictions, was employed to evaluate their therapeutic potential. DFT calculations at the B3LYP/def2-TZVP level revealed key electronic properties, with TD3 compound demonstrating the highest chemical reactivity. Molecular Electrostatic Potential (MEP) and Reduced Density Gradient (RDG) analyses provided insights into the compounds' non-covalent interactions and charge distributions. Molecular docking studies against the NS5 protein (PDB: 6KR2) showed superior binding affinities for all three compounds compared to the control ligand SAH, with TD3 exhibiting the lowest binding energy (−8.41 kcal/mol) and theoretical inhibition constant (689.31 nM). ADME predictions indicated favorable drug-like properties with concerns regarding aqueous solubility and potential P-glycoprotein interactions. Toxicity evaluations highlighted challenges, particularly in hepatotoxicity and carcinogenicity. The study identified TD3 as a promising lead compound for Dengue Virus NS5 inhibition, while also emphasizing the need for targeted modifications to address toxicity concerns. This research not only contributes to anti-dengue drug discovery efforts but also provides a robust methodological framework for the theoretical evaluation of similar small compounds in future investigations.
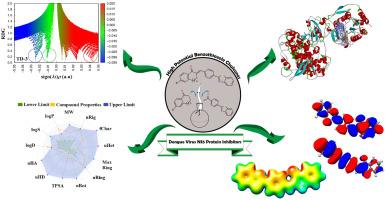
利用高潜力苯并噻唑查尔酮对抗登革热病毒 NS5 蛋白:通过分子对接、ADME 和 DFT 进行的多方面理论研究。
采用一般的克莱森-施密特缩合方案,成功合成了以四氢酮、茚酮和苯并噻唑为核心的查耳酮。制备的化合物经过纯化,并通过 1H、13C-NMR 和 FT-IR 技术进行了结构分析。研究人员采用密度泛函理论(DFT)、分子对接和 ADME 预测相结合的多元理论方法来评估这些化合物的治疗潜力。在 B3LYP/def2-TZVP 水平上进行的 DFT 计算揭示了关键的电子特性,其中 TD3 化合物的化学反应活性最高。分子静电位(MEP)和还原密度梯度(RDG)分析深入揭示了化合物的非共价相互作用和电荷分布。针对 NS5 蛋白(PDB:6KR2)的分子对接研究表明,与对照配体 SAH 相比,所有三种化合物都具有更优越的结合亲和力,其中 TD3 的结合能(-8.41 kcal/mol)和理论抑制常数(689.31 nM)最低。ADME 预测表明,TD3 具有良好的类药物特性,但水溶性和潜在的 P 糖蛋白相互作用令人担忧。毒性评估凸显了挑战,尤其是肝毒性和致癌性。研究发现 TD3 是一种很有前景的登革病毒 NS5 抑制先导化合物,同时也强调了有必要进行有针对性的改造,以解决毒性问题。这项研究不仅有助于抗登革热药物的发现工作,还为今后研究中类似小化合物的理论评估提供了强有力的方法框架。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


