Serum metabolite biomarkers for the early diagnosis and monitoring of age-related macular degeneration
IF 11.4
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Introduction
Age-related macular degeneration (AMD) is a leading cause of irreversible blindness worldwide, with significant challenges for early diagnosis and treatment.Objectives
To identify new biomarkers that are important for the early diagnosis and monitoring of the severity/progression of AMD.Methods
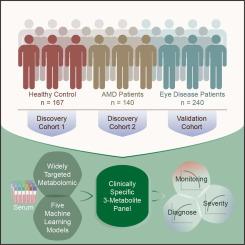
We investigated the diagnostic and monitoring potential of blood metabolites in a cohort of 547 individuals (167 healthy controls, 240 individuals with other eye diseases as eye disease controls, and 140 individuals with AMD) from 2 centers over three phases: discovery phase 1, discovery phase 2, and an external validation phase. The samples were analyzed via a mass spectrometry-based, widely targeted metabolomic workflow. In discovery phases 1 and 2, we built a machine learning algorithm to predict the probability of AMD. In the external validation phase, we further confirmed the performance of the biomarker panel identified by the algorithm. We subsequently evaluated the performance of the identified biomarker panel in monitoring the progression and severity of AMD.Results
We developed a clinically specific three-metabolite panel (hypoxanthine, 2-furoylglycine, and 1-hexadecyl-2-azelaoyl-sn-glycero-3-phosphocholine) via five machine learning models. The random forest model effectively discriminated patients with AMD from patents in the other two groups and showed acceptable calibration (area under the curve (AUC) = 1.0; accuracy = 1.0) in both discovery phases 1 and 2. An independent validation phase confirmed the diagnostic model’s efficacy (AUC = 0.962; accuracy = 0.88). The three-biomarker panel model demonstrated an AUC of 1.0 in differentiating the severity of AMD via RF machine learning, which was consistent across both the discovery and external validation phases. Additionally, the biomarker concentrations remained stable under repeated freeze–thaw cycles (P > 0.05).Conclusions
This study reveals distinct metabolite variations in the serum of AMD patients, paving the way for the development of the first routine laboratory test for AMD.
用于早期诊断和监测老年性黄斑变性的血清代谢物生物标记物
方法 我们对来自 2 个中心的 547 人队列(167 名健康对照组、240 名其他眼病对照组和 140 名 AMD 患者)的血液代谢物的诊断和监测潜力进行了调查,调查分为三个阶段:发现阶段 1、发现阶段 2 和外部验证阶段。样本通过基于质谱的广泛靶向代谢组学工作流程进行分析。在发现阶段 1 和 2,我们建立了一种机器学习算法来预测 AMD 的概率。在外部验证阶段,我们进一步确认了该算法确定的生物标记物面板的性能。结果我们通过五个机器学习模型建立了一个临床特异性的三代谢物面板(次黄嘌呤、2-呋喃基甘氨酸和1-十六烷基-2-氮杂酰-sn-甘油-3-磷酸胆碱)。随机森林模型能有效区分 AMD 患者和其他两组患者,并在发现阶段 1 和 2 显示出可接受的校准(曲线下面积 (AUC) = 1.0;准确度 = 1.0)。独立验证阶段证实了诊断模型的有效性(AUC = 0.962;准确度 = 0.88)。通过射频机器学习,三生物标志物面板模型在区分老年性视网膜病变严重程度方面的AUC为1.0,这在发现阶段和外部验证阶段都是一致的。结论这项研究揭示了 AMD 患者血清中代谢物的明显变化,为开发首个 AMD 常规实验室检验铺平了道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Advanced Research
Multidisciplinary-Multidisciplinary
CiteScore
21.60
自引率
0.90%
发文量
280
审稿时长
12 weeks
期刊介绍:
Journal of Advanced Research (J. Adv. Res.) is an applied/natural sciences, peer-reviewed journal that focuses on interdisciplinary research. The journal aims to contribute to applied research and knowledge worldwide through the publication of original and high-quality research articles in the fields of Medicine, Pharmaceutical Sciences, Dentistry, Physical Therapy, Veterinary Medicine, and Basic and Biological Sciences.
The following abstracting and indexing services cover the Journal of Advanced Research: PubMed/Medline, Essential Science Indicators, Web of Science, Scopus, PubMed Central, PubMed, Science Citation Index Expanded, Directory of Open Access Journals (DOAJ), and INSPEC.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


