Asymmetric Hydrogenation of Naphthalenes with Molybdenum Catalysts: Ligand Design Improves Chemoselectivity
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The asymmetric hydrogenation of substituted naphthalenes with a series of highly enantioenriched oxazoline imino(pyridine) (OIP) molybdenum cyclooctadiene precatalysts is described. The chemoselectivity of the hydrogenation for the formation of tetralin versus decalin products was systematically explored as a function of the aniline substituents on the molybdenum precatalysts. Examples with 2,6-disubstitution with methyl, ethyl, or iso-propyl substituents were the most active but produced near equimolar mixtures of tetralins and decalins, both with high enantiomeric excesses. Introduction of anilines with either 2-tert-butyl or 2,5-di-tert-butyl substituents increased the selectivity for decalin formation albeit with reduced overall hydrogenation activity. In all cases with 2,6-disubtituted naphthalenes, high enantiomeric excesses are observed. Mechanistic studies demonstrated that [(OIP)Mo] catalysts are inactive for tetralin hydrogenation and analysis of the catalytic reactions by NMR spectroscopy, in combination with independent synthesis, identified (OIP)Mo(η6-naphthalene) complexes as the catalyst resting states. Assaying product selectivity as a function of catalyst loading established that increasing the amount of molybdenum precatalyst increased the selectivity for decalin formation. Substrate concentration studies were also conducted and support a pathway whereby tetralin formation arises from displacement of the arene from the coordination sphere of the molybdenum by incoming substrate. These studies establish general design principals for [(OIP)Mo] arene hydrogenation catalysts where larger 2,6-aniline substituents increase activity, likely by promoting η6 to η4 haptotropic rearrangement from the arene resting state, while single tert-butyl substitution promotes more selective hydrogenation to decalin products.
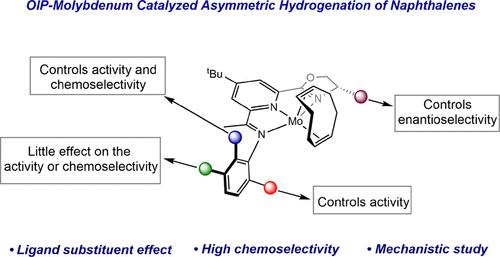
钼催化剂的萘不对称加氢反应:配体设计提高化学选择性
本研究介绍了一系列高对映体富集的噁唑啉亚氨基(吡啶)(OIP)钼环辛二烯前催化剂对取代萘的不对称氢化反应。我们系统地探讨了加氢生成四氢萘酚和癸萘酚产物的化学选择性与钼前催化剂上苯胺取代基的函数关系。具有甲基、乙基或异丙基取代基的 2,6 二甲基苯胺是最活跃的例子,但生成的四氢萘和癸萘酚混合物接近等摩尔,对映体过量率都很高。引入具有 2-叔丁基或 2,5-二叔丁基取代基的苯胺会提高癸醛的生成选择性,但总体氢化活性会降低。在所有 2,6-二取代萘的情况下,都观察到对映体的高过量。机理研究表明,[(OIP)Mo] 催化剂对四氢萘无活性,通过核磁共振光谱分析催化反应并结合独立合成,确定 (OIP)Mo(η6-萘) 复合物为催化剂静止状态。通过测定产物选择性与催化剂负载量的函数关系,确定了增加钼前催化剂的用量可提高癸醛形成的选择性。此外,还进行了底物浓度研究,结果表明,四氢萘的形成是由于钼的配位球中的炔烃被进入的底物置换所致。这些研究确立了[(OIP)Mo]炔氢化催化剂的一般设计原则,其中较大的 2,6-苯胺取代基提高了活性,这可能是通过促进从炔静止态的η6 到η4 的触变重排,而单一的叔丁基取代则促进更有选择性地氢化为蜕皮激素产物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


