mRNA vaccines for infectious diseases — advances, challenges and opportunities
IF 122.7
1区 医学
Q1 BIOTECHNOLOGY & APPLIED MICROBIOLOGY
引用次数: 0
Abstract
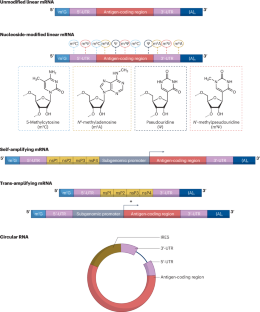
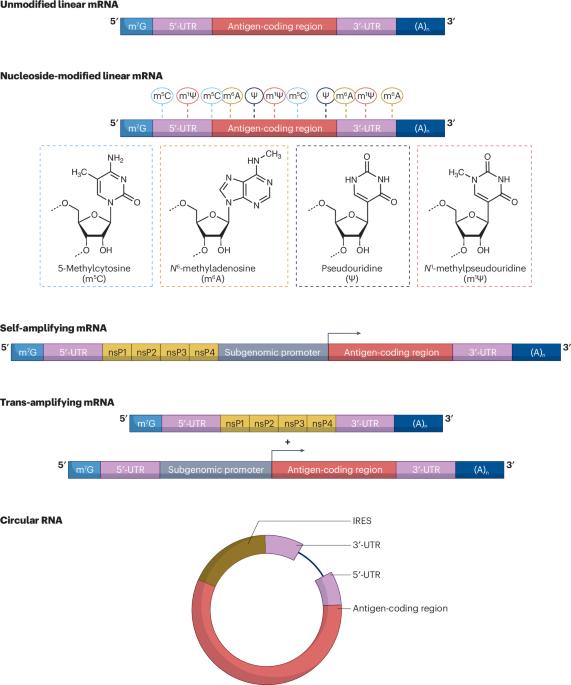
The concept of mRNA-based vaccines emerged more than three decades ago. Groundbreaking discoveries and technological advancements over the past 20 years have resolved the major roadblocks that initially delayed application of this new vaccine modality. The rapid development of nucleoside-modified COVID-19 mRNA vaccines demonstrated that this immunization platform is easy to develop, has an acceptable safety profile and can be produced at a large scale. The flexibility and ease of antigen design have enabled mRNA vaccines to enter development for a wide range of viruses as well as for various bacteria and parasites. However, gaps in our knowledge limit the development of next-generation mRNA vaccines with increased potency and safety. A deeper understanding of the mechanisms of action of mRNA vaccines, application of novel technologies enabling rational antigen design, and innovative vaccine delivery strategies and vaccination regimens will likely yield potent novel vaccines against a wide range of pathogens. Following the success of the COVID-19 vaccines, mRNA vaccines have now entered development for a wide range of infectious diseases. This Review discusses mRNA vaccine design considerations, delivery strategies and mechanisms of action, assessing mRNA vaccines currently in development for various viruses, bacteria and parasites. The challenges, limitations and opportunities facing next-generation mRNA vaccines are considered.
用于传染病的 mRNA 疫苗--进展、挑战和机遇
基于 mRNA 的疫苗概念出现于三十多年前。过去 20 年中的突破性发现和技术进步解决了最初阻碍这种新型疫苗应用的主要障碍。核苷修饰的 COVID-19 mRNA 疫苗的快速开发表明,这种免疫平台易于开发,具有可接受的安全性,并且可以大规模生产。抗原设计的灵活性和简易性使 mRNA 疫苗能够用于多种病毒以及各种细菌和寄生虫的开发。然而,我们的知识空白限制了效力和安全性更高的下一代 mRNA 疫苗的开发。深入了解 mRNA 疫苗的作用机制,应用新技术合理设计抗原,以及创新的疫苗递送策略和接种方案,将有可能开发出针对多种病原体的强效新型疫苗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Reviews. Drug Discovery
医学-生物工程与应用微生物
CiteScore
137.40
自引率
0.30%
发文量
227
期刊介绍:
Nature Reviews Drug Discovery is a monthly journal aimed at everyone working in the drug discovery and development arena.
Each issue includes:
Highest-quality reviews and perspectives covering a broad scope.
News stories investigating the hottest topics in drug discovery.
Timely summaries of key primary research papers.
Concise updates on the latest advances in areas such as new drug approvals, patent law, and emerging industry trends and strategies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


