T lymphocyte recruitment to melanoma brain tumors depends on distinct venous vessels
IF 25.5
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
To improve immunotherapy for brain tumors, it is important to determine the principal intracranial site of T cell recruitment from the bloodstream and their intracranial route to brain tumors. Using intravital microscopy in mouse models of intracranial melanoma, we discovered that circulating T cells preferably adhered and extravasated at a distinct type of venous blood vessel in the tumor vicinity, peritumoral venous vessels (PVVs). Other vascular structures were excluded as alternative T cell routes to intracranial melanomas. Anti-PD-1/CTLA-4 immune checkpoint inhibitors increased intracranial T cell motility, facilitating migration from PVVs to the tumor and subsequently inhibiting intracranial tumor growth. The endothelial adhesion molecule ICAM-1 was particularly expressed on PVVs, and, in samples of human brain metastases, ICAM-1 positivity of PVV-like vessels correlated with intratumoral T cell infiltration. These findings uncover a distinct mechanism by which the immune system can access and control brain tumors and potentially influence other brain pathologies.
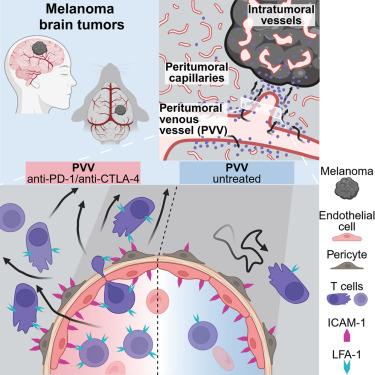
黑色素瘤脑肿瘤的 T 淋巴细胞募集依赖于独特的静脉血管
为了改善脑肿瘤的免疫疗法,必须确定T细胞从血液招募到脑肿瘤的主要颅内部位及其颅内途径。通过在颅内黑色素瘤小鼠模型中使用体视显微镜,我们发现循环中的T细胞最好附着在肿瘤附近一种独特的静脉血管--瘤周静脉血管(PVVs)上并外渗。其他血管结构被排除在T细胞进入颅内黑色素瘤的替代途径之外。抗PD-1/CTLA-4免疫检查点抑制剂增加了颅内T细胞的运动性,促进了从瘤周静脉血管向肿瘤的迁移,进而抑制了颅内肿瘤的生长。内皮粘附分子ICAM-1在PVV上的表达尤为突出,在人类脑转移瘤样本中,PVV样血管的ICAM-1阳性与瘤内T细胞浸润相关。这些发现揭示了免疫系统进入和控制脑肿瘤并可能影响其他脑部病变的独特机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Immunity
医学-免疫学
CiteScore
49.40
自引率
2.20%
发文量
205
审稿时长
6 months
期刊介绍:
Immunity is a publication that focuses on publishing significant advancements in research related to immunology. We encourage the submission of studies that offer groundbreaking immunological discoveries, whether at the molecular, cellular, or whole organism level. Topics of interest encompass a wide range, such as cancer, infectious diseases, neuroimmunology, autoimmune diseases, allergies, mucosal immunity, metabolic diseases, and homeostasis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


