Predicting protein interactions of the kinase Lck critical to T cell modulation
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
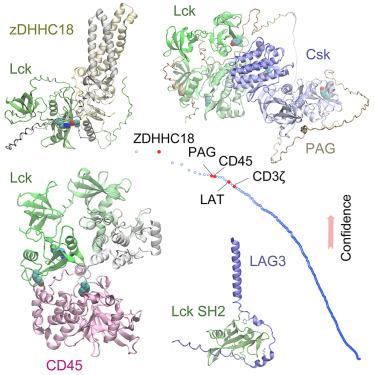
Protein-protein interactions (PPIs) play pivotal roles in directing T cell fate. One key player is the non-receptor tyrosine protein kinase Lck that helps to transduce T cell activation signals. Lck is mediated by other proteins via interactions that are inadequately understood. Here, we use the deep learning method AF2Complex to predict PPIs involving Lck, by screening it against ∼1,000 proteins implicated in immune responses, followed by extensive structural modeling for selected interactions. Remarkably, we describe how Lck may be specifically targeted by a palmitoyltransferase using a phosphotyrosine motif. We uncover “hotspot” interactions between Lck and the tyrosine phosphatase CD45, leading to a significant conformational shift of Lck for activation. Lastly, we present intriguing interactions between the phosphotyrosine-binding domain of Lck and the cytoplasmic tail of the immune checkpoint LAG3 and propose a molecular mechanism for its inhibitory role. Together, this multifaceted study provides valuable insights into T cell regulation and signaling.
预测对 T 细胞调控至关重要的激酶 Lck 蛋白相互作用
蛋白质与蛋白质之间的相互作用(PPIs)在指导 T 细胞命运方面发挥着关键作用。其中一个关键角色是非受体酪氨酸蛋白激酶 Lck,它有助于传递 T 细胞活化信号。Lck 是由其他蛋白质通过相互作用介导的,而人们对这些相互作用的了解还很不够。在这里,我们使用深度学习方法 AF2Complex 预测涉及 Lck 的 PPIs,方法是将其与∼1,000 个与免疫反应有关的蛋白质进行筛选,然后对选定的相互作用进行广泛的结构建模。值得注意的是,我们描述了棕榈酰基转移酶如何利用磷酸酪氨酸基团特异性地靶向 Lck。我们发现了 Lck 与酪氨酸磷酸酶 CD45 之间的 "热点 "相互作用,从而导致 Lck 在激活时发生显著的构象转变。最后,我们展示了 Lck 的磷酸酪氨酸结合域与免疫检查点 LAG3 胞质尾之间有趣的相互作用,并提出了其抑制作用的分子机制。总之,这项多方面的研究为 T 细胞调控和信号传导提供了宝贵的见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


