Boosting chondrocyte bioactivity with ultra-sulfated glycopeptide supramolecular polymers
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
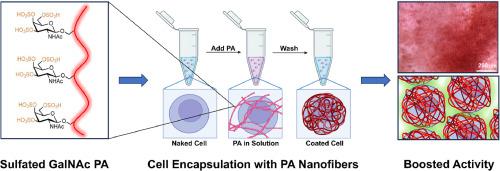
Although autologous chondrocyte transplantation can be effective in articular cartilage repair, negative side effects limit the utility of the treatment, such as long recovery times, poor engraftment or chondrogenic dedifferentiation, and cell leakage. Peptide-based supramolecular polymers have emerged as promising bioactive systems to promote tissue regeneration through cell signaling and dynamic behavior. We report here on the development of a series of glycopeptide amphiphile supramolecular nanofibers with chondrogenic bioactivity. These supramolecular polymers were found to have the ability to boost TGFβ-1 signaling by displaying galactosamine moieties with differing degrees of sulfation on their surfaces. We were also able to encapsulate chondrocytes with these nanostructures as single cells without affecting viability and proliferation. Among the monomers tested, assemblies of trisulfated glycopeptides led to elevated expression of chondrogenic markers relative to those with lower degrees of sulfation that mimic chondroitin sulfate repeating units. We hypothesize the enhanced bioactivity is rooted in specific interactions of the supramolecular assemblies with TGFβ-1 and its consequence on cell signaling, which may involve elevated levels of supramolecular motion as a result of high charge in trisulfated glycopeptide amphiphiles. Our findings suggest that supramolecular polymers formed by the ultra-sulfated glycopeptide amphiphiles could provide better outcomes in chondrocyte transplantation therapies for cartilage regeneration.
Statement of significance
This study prepares glycopeptide amphiphiles conjugated at their termini with chondroitin sulfate mimetic residues with varying degrees of sulfation that self-assemble into supramolecular nanofibers in aqueous solution. These supramolecular polymers encapsulate chondrocytes as single cells through intimate contact with cell surface structures, forming artificial matrix that can localize the growth factor TGFβ-1 in the intercellular environment. A high degree of sulfation on the glycopeptide amphiphile is found to be critical in elevating chondrogenic cellular responses that supersede the efficacy of natural chondroitin sulfate. This work demonstrates that supramolecular assembly of a unique molecular structure designed to mimic chondroitin sulfate successfully boosts chondrocyte bioactivity by single cell encapsulation, suggesting a new avenue implementing chondrocyte transplantation with supramolecular nanomaterials for cartilage regeneration.
利用超硫酸化甘肽超分子聚合物提高软骨细胞的生物活性
虽然自体软骨细胞移植可有效修复关节软骨,但其负面影响限制了该疗法的实用性,如恢复时间长、接合不良或软骨源性去分化以及细胞渗漏。基于肽的超分子聚合物已成为一种很有前景的生物活性系统,可通过细胞信号传导和动态行为促进组织再生。我们在此报告了一系列具有软骨生物活性的糖肽双亲超分子纳米纤维的开发情况。研究发现,这些超分子聚合物通过在其表面显示不同硫酸化程度的半乳糖胺分子,具有促进 TGFβ-1 信号传导的能力。我们还能用这些纳米结构将软骨细胞包裹成单细胞,而不影响其活力和增殖。在测试的单体中,相对于硫酸软骨素重复单元的硫酸化程度较低的单体,三硫酸化糖肽的集合体可提高软骨生成标记物的表达。我们推测,生物活性的增强源于超分子组装体与 TGFβ-1 的特定相互作用及其对细胞信号传导的影响,这可能涉及到三硫化糖肽两亲化合物中的高电荷导致的超分子运动水平升高。我们的研究结果表明,超硫酸化糖肽两亲化合物形成的超分子聚合物可为软骨再生的软骨细胞移植疗法提供更好的结果。重要意义本研究制备了在其端部与不同硫酸化程度的硫酸软骨素模拟残基共轭的糖肽两亲化合物,这些糖肽两亲化合物可在水溶液中自组装成超分子纳米纤维。这些超分子聚合物通过与细胞表面结构的亲密接触,将软骨细胞作为单细胞包裹起来,形成人工基质,可将生长因子 TGFβ-1 定位于细胞间环境中。研究发现,糖肽两性体上的高度硫酸化是提高软骨细胞反应的关键,这种反应可超越天然硫酸软骨素的功效。这项研究表明,超分子组装的独特分子结构旨在模仿硫酸软骨素,通过单细胞包裹成功提高了软骨细胞的生物活性,为利用超分子纳米材料进行软骨细胞移植促进软骨再生开辟了一条新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


