Inhibition of phototrophic iron oxidation by nitric oxide in ferruginous environments
IF 15.7
1区 地球科学
Q1 GEOSCIENCES, MULTIDISCIPLINARY
引用次数: 0
Abstract
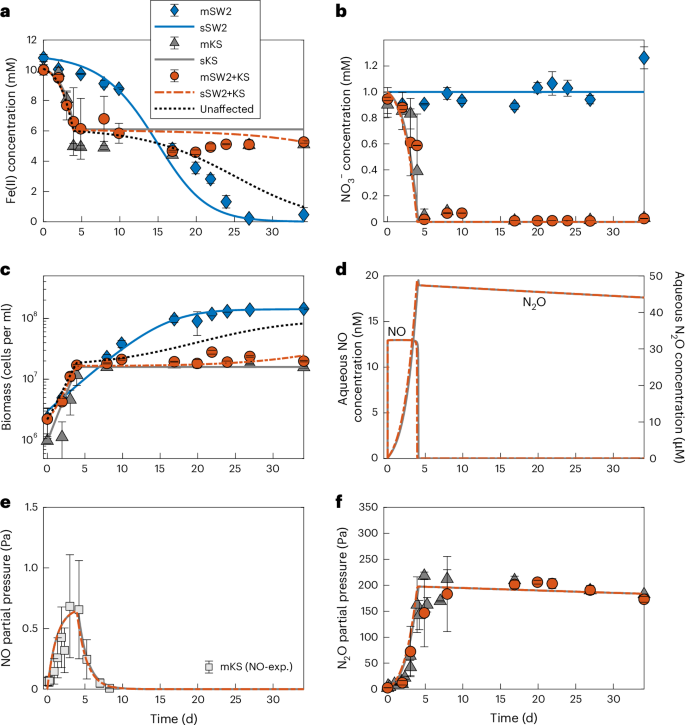
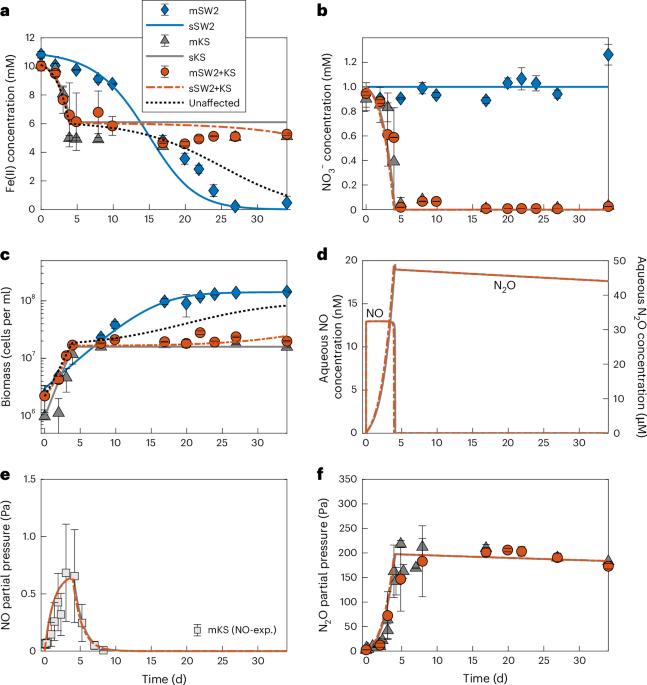
Anoxygenic phototrophic Fe(II) oxidizers (photoferrotrophs) are thought to have thrived in Earth’s ancient ferruginous oceans and played a primary role in the precipitation of Archaean and Palaeoproterozoic (3.8–1.85-billion-year-old) banded iron formations (BIFs). The end of BIF deposition by photoferrotrophs has been interpreted as the result of a deepening of water-column oxygenation below the photic zone, concomitant with the proliferation of cyanobacteria. However, photoferrotrophs may have experienced competition from other anaerobic Fe(II)-oxidizing microorganisms, altering the formation mechanism of BIFs. Here we utilize microbial incubations to show that nitrate-reducing Fe(II) oxidizers metabolically outcompete photoferrotrophs for dissolved Fe(II). Moreover, both experiments and numerical modelling show that the nitrate-reducing Fe(II) oxidizers inhibit photoferrotrophy via the production of toxic intermediates. Four different photoferrotrophs, representing both green sulfur and purple non-sulfur bacteria, are susceptible to this toxic effect despite having genomic capabilities for nitric oxide detoxification. Indeed, despite nitric oxide detoxification mechanisms being ubiquitous in some groups of phototrophs at the genomic level (for example, Chlorobi and Cyanobacteria) it is likely that they would still be affected. We suggest that the production of reactive nitrogen species during nitrate-reducing Fe(II) oxidation in ferruginous environments may have inhibited the activity of photoferrotrophs in the ancient oceans and thus impeded their role in the precipitation of BIFs. Banded iron formation deposition by photoferrotrophic organisms in the early Earth’s oceans may have been inhibited by competition for iron and toxicity from nitrate-reducing microorganisms, according to a microbial incubation and numerical modelling study.
一氧化氮对铁质环境中光养铁氧化的抑制作用
据认为,无氧光养铁(II)氧化物(光铁藻)曾在地球的古铁锈海洋中繁衍生息,并在太古宙和古近纪(38-18.5 亿年前)带状铁层(BIFs)的沉淀过程中发挥了主要作用。光铁锈层沉积的结束被解释为光照区以下水柱含氧量加深以及蓝藻大量繁殖的结果。然而,光能嗜水生物可能经历了来自其他厌氧铁(II)氧化微生物的竞争,从而改变了 BIFs 的形成机制。在这里,我们利用微生物培养方法证明,硝酸盐还原型铁(II)氧化剂在代谢过程中会比光铁嗜水菌更容易获得溶解的铁(II)。此外,实验和数值模拟都表明,硝酸盐还原型铁(II)氧化剂通过产生有毒的中间产物来抑制光铁锈作用。代表绿色硫细菌和紫色非硫细菌的四种不同的光铁锈营养体,尽管基因组具有一氧化氮解毒能力,但都容易受到这种毒性效应的影响。事实上,尽管一氧化氮解毒机制在某些光养菌群(如绿僵菌和蓝藻)的基因组水平上无处不在,但它们仍有可能受到影响。我们认为,铁锈质环境中硝酸盐还原铁(II)氧化过程中产生的活性氮物种可能抑制了古海洋中光铁细菌的活性,从而阻碍了它们在 BIFs 沉淀过程中发挥作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Geoscience
地学-地球科学综合
CiteScore
26.70
自引率
1.60%
发文量
187
审稿时长
3.3 months
期刊介绍:
Nature Geoscience is a monthly interdisciplinary journal that gathers top-tier research spanning Earth Sciences and related fields.
The journal covers all geoscience disciplines, including fieldwork, modeling, and theoretical studies.
Topics include atmospheric science, biogeochemistry, climate science, geobiology, geochemistry, geoinformatics, remote sensing, geology, geomagnetism, paleomagnetism, geomorphology, geophysics, glaciology, hydrology, limnology, mineralogy, oceanography, paleontology, paleoclimatology, paleoceanography, petrology, planetary science, seismology, space physics, tectonics, and volcanology.
Nature Geoscience upholds its commitment to publishing significant, high-quality Earth Sciences research through fair, rapid, and rigorous peer review, overseen by a team of full-time professional editors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


