Hydrophobic assembly of molecular catalysts at the gas–liquid–solid interface drives highly selective CO2 electromethanation
IF 19.2
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
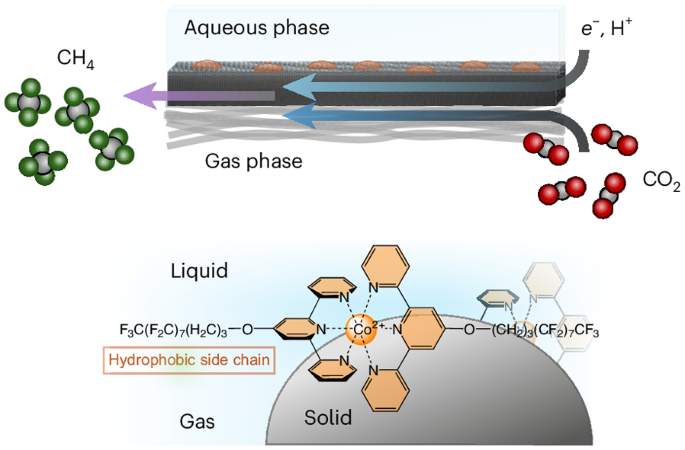
Molecular catalysts offer tunable active and peripheral sites, rendering them ideal model systems to explore fundamental concepts in catalysis. However, hydrophobic designs are often regarded as detrimental for dissolution in aqueous electrolytes. Here we show that established cobalt terpyridine catalysts modified with hydrophobic perfluorinated alkyl side chains can assemble at the gas–liquid–solid interfaces on a gas diffusion electrode. We find that the self-assembly of these perfluorinated units on the electrode surface results in a catalytic system selective for electrochemical CO2 reduction to CH4, whereas every other cobalt terpyridine catalyst reported previously was only selective for CO or formate. Mechanistic investigations suggest that the pyridine units function as proton shuttles that deliver protons to the dynamic hydrophobic pocket in which CO2 reduction takes place. Finally, integration with fluorinated carbon nanotubes as a hydrophobic conductive scaffold leads to a Faradaic efficiency for CH4 production above 80% at rates above 10 mA cm−2—impressive activities for a molecular electrocatalytic system. Although molecular complexes can serve as well-defined model catalysts for CO2 electroreduction, few compounds reduce CO2 beyond two electrons. Now, hydrophobic molecular cobalt terpyridine complexes, containing perfluorinated alkyl side chains, have been shown to assemble at the gas–liquid–solid interface and to electrocatalytically reduce CO2 to methane with high efficiencies.

气-液-固界面上分子催化剂的疏水装配推动了高选择性二氧化碳电甲烷化
分子催化剂具有可调的活性和外围位点,是探索催化基本概念的理想模型系统。然而,疏水性设计通常被认为不利于在水性电解质中的溶解。在这里,我们展示了用疏水性全氟烷基侧链修饰的钴三联吡啶催化剂可以在气体扩散电极的气-液-固界面上组装。我们发现,这些全氟单元在电极表面的自组装导致催化系统对电化学 CO2 还原成 CH4 具有选择性,而之前报道的其他所有钴萜吡啶催化剂都只对 CO 或甲酸盐具有选择性。机理研究表明,吡啶单元具有质子穿梭器的功能,可将质子输送到发生 CO2 还原的动态疏水口袋中。最后,与作为疏水性导电支架的氟化碳纳米管相结合,在速率超过 10 mA cm-2 的情况下,产生 CH4 的法拉第效率超过 80%--这对于分子电催化系统来说是令人印象深刻的。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature chemistry
化学-化学综合
CiteScore
29.60
自引率
1.40%
发文量
226
审稿时长
1.7 months
期刊介绍:
Nature Chemistry is a monthly journal that publishes groundbreaking and significant research in all areas of chemistry. It covers traditional subjects such as analytical, inorganic, organic, and physical chemistry, as well as a wide range of other topics including catalysis, computational and theoretical chemistry, and environmental chemistry.
The journal also features interdisciplinary research at the interface of chemistry with biology, materials science, nanotechnology, and physics. Manuscripts detailing such multidisciplinary work are encouraged, as long as the central theme pertains to chemistry.
Aside from primary research, Nature Chemistry publishes review articles, news and views, research highlights from other journals, commentaries, book reviews, correspondence, and analysis of the broader chemical landscape. It also addresses crucial issues related to education, funding, policy, intellectual property, and the societal impact of chemistry.
Nature Chemistry is dedicated to ensuring the highest standards of original research through a fair and rigorous review process. It offers authors maximum visibility for their papers, access to a broad readership, exceptional copy editing and production standards, rapid publication, and independence from academic societies and other vested interests.
Overall, Nature Chemistry aims to be the authoritative voice of the global chemical community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


