Effect of 4,4′-dialkyl-2,2′-bipyridine ligands on the hydrolysis of dichlorodioxomolybdenum(VI) catalyst precursors and the switch from homogeneous epoxidation to heterogeneous systems
IF 6.5
1区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
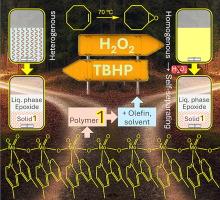
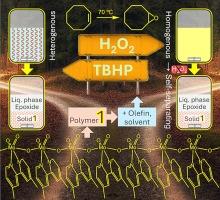
Molybdenum catalysts have been industrially recognized for decades for liquid phase epoxidation, which is an important chemical reaction process, since epoxides are used for many industrial applications. In this work, molybdenum oxide hybrid catalysts, prepared by a reflux hydrolysis methodology, performed effectively as heterogeneous catalysts or reaction-induced self-separating catalysts under mild reaction conditions; in the two cases, the catalyst separation and reuse are facilitated. Specifically, catalysts with the general formula [MoO3(L)], possessing polymeric chain-like (L = 4,4′-dimethyl-2,2′-bipyridine (1)) or oligomeric (L = 4,4′-dinonyl-2,2′-bipyridine (2)) structures comprising corner-sharing {MoO4N2} units, were synthesized and characterized by various complementary techniques (ATR FT-IR, Raman, 13C{1H} CP MAS NMR spectroscopy, PXRD, SEM, TGA, elemental analysis, ICP-OES and N2 sorption isotherms). Small chemical differences in the organic synthesis precursor had important structure directing effects on the type of hybrid material formed. The hybrids promoted olefin epoxidation with H2O2 or tert-butylhydroperoxide (TBHP) as oxidant. For example, 1 catalyzed the conversion of biobased olefins (70 °C) and lignin-based isoeugenol (50 °C) with TBHP to useful bioproducts, in heterogeneous phase, leading to an epoxide yield of 100 % for DL-limonene (3:1 M molar ratio of 1,2-epoxy-p-menth-8-ene to 1,2:8,9-diepoxy-p-menthane), 81 % epoxide yield for fatty acid methyl esters, and 80 % Licarin A selectivity at 40 % isoeugenol conversion. For dienes (DL-limonene, methyl linoleate), kinetic modelling studies suggested that the formation of the monoepoxides was faster than that of diepoxides, accounting for enhanced monoepoxide selectivity.
4,4′-二烷基-2,2′-联吡啶配体对二氯二氧代钼(VI)催化剂前体水解的影响以及从均相环氧化反应到异相体系的转换
几十年来,钼催化剂在液相环氧化中的应用已得到工业界的认可,环氧化是一种重要的化学反应过程,因为环氧化物在许多工业领域都有应用。在这项工作中,采用回流水解法制备的氧化钼杂化催化剂在温和的反应条件下作为异相催化剂或反应诱导自分离催化剂发挥了有效的作用;在这两种情况下,催化剂的分离和重复使用都很方便。具体来说,通式为[MoO3(L)]的催化剂具有聚合链状(L = 4,4′-二甲基-2,2′-联吡啶 (1))或低聚(L = 4,4′-二壬基-2、2′-bipyridine (2))结构,并通过各种互补技术(ATR 傅立叶变换红外光谱、拉曼光谱、13C{1H} CP MAS 核磁共振光谱)进行表征。CP MAS NMR 光谱、PXRD、SEM、TGA、元素分析、ICP-OES 和 N2 吸附等温线)。有机合成前体中的微小化学差异对所形成的杂化材料类型具有重要的结构导向作用。以 H2O2 或叔丁基过氧化氢(TBHP)为氧化剂的混合物可促进烯烃环氧化反应。例如,1 与 TBHP 在异相催化生物基烯烃(70 °C)和木质素基异丁香酚(50 °C)转化为有用的生物产品,DL-柠檬烯的环氧化物产率达到 100%(3:1,2-epoxy-p-menth-8-ene 与 1,2:8,9-diepoxy-p-menthane)的比例为 1 M,脂肪酸甲酯的环氧化物产率为 81%,异丁香酚转化率为 40%时,利卡灵 A 的选择性为 80%。对于二烯(DL-柠檬烯、亚油酸甲酯),动力学模型研究表明,单环氧化物的形成速度快于二环氧化物,这也是单环氧化物选择性增强的原因。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Catalysis
工程技术-工程:化工
CiteScore
12.30
自引率
5.50%
发文量
447
审稿时长
31 days
期刊介绍:
The Journal of Catalysis publishes scholarly articles on both heterogeneous and homogeneous catalysis, covering a wide range of chemical transformations. These include various types of catalysis, such as those mediated by photons, plasmons, and electrons. The focus of the studies is to understand the relationship between catalytic function and the underlying chemical properties of surfaces and metal complexes.
The articles in the journal offer innovative concepts and explore the synthesis and kinetics of inorganic solids and homogeneous complexes. Furthermore, they discuss spectroscopic techniques for characterizing catalysts, investigate the interaction of probes and reacting species with catalysts, and employ theoretical methods.
The research presented in the journal should have direct relevance to the field of catalytic processes, addressing either fundamental aspects or applications of catalysis.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


