Non-fluorinated electrolytes with micelle-like solvation for ultra-high-energy-density lithium metal batteries
IF 19.1
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
Electrolyte engineering plays a critical role in enabling lithium (Li) metal batteries. However, the simultaneous realization of anion-rich solvation structure and high ionic conductivity of electrolytes via solvation structure design remains challenging. Here, we report a low-cost, non-fluorinated electrolyte with a micelle-like solvation structure by introducing amphiphilic n-butyl methyl ether (MNBE) into Li bis(fluorosulfonyl)imide (LiFSI)/1,2-dimethoxyethane (DME) for stable Li metal batteries (LMBs). MNBE can effectively promote Li+-FSI− coordination through steric crowding. Meanwhile, the inert alkyl chains of MNBE can mitigate the reaction between electrolyte and Li metal due to their lithiophobicity. Specifically, the micelle-like, non-fluorinated electrolyte exhibits an ionic conductivity as high as 12.55 mS cm−1, and its anion-rich solvation structure promotes the formation of LiF-rich solid-electrolyte interphase. We constructed a 7.3 Ah Li||NMC811 pouch cell employing this electrolyte under harsh conditions, exhibiting ultra-high specific energy of 503.7 Wh kg−1 with impressive cycling stability of 84.1% capacity retention after 100 cycles.
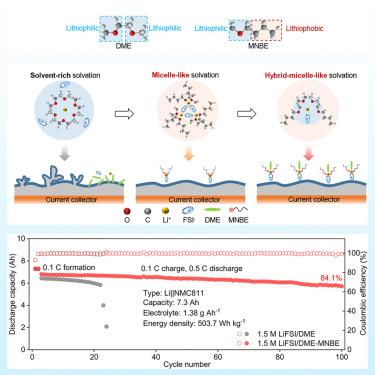

用于超高能量密度锂金属电池的胶束状溶解的无氟电解质
电解质工程在实现锂(Li)金属电池方面发挥着至关重要的作用。然而,通过溶解结构设计同时实现电解质的富阴离子溶解结构和高离子电导率仍然具有挑战性。在此,我们通过在双氟磺酰亚胺锂(LiFSI)/1,2-二甲氧基乙烷(DME)中引入两亲性正丁基甲基醚(MNBE),报告了一种具有胶束状溶解结构的低成本无氟电解质,可用于制造稳定的锂金属电池(LMB)。MNBE 可通过立体拥挤有效促进 Li+-FSI- 配位。同时,MNBE 的惰性烷基链具有疏锂性,可减轻电解质与锂金属之间的反应。具体来说,胶束状非氟化电解质的离子电导率高达 12.55 mS cm-1,其富含阴离子的溶解结构促进了富含 LiF 的固体-电解质间相的形成。我们在苛刻的条件下利用这种电解质构建了一个 7.3 Ah 的锂||NMC811 袋式电池,显示出 503.7 Wh kg-1 的超高比能量和令人印象深刻的循环稳定性,100 次循环后容量保持率为 84.1%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chem
Environmental Science-Environmental Chemistry
CiteScore
32.40
自引率
1.30%
发文量
281
期刊介绍:
Chem, affiliated with Cell as its sister journal, serves as a platform for groundbreaking research and illustrates how fundamental inquiries in chemistry and its related fields can contribute to addressing future global challenges. It was established in 2016, and is currently edited by Robert Eagling.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


