A Highly Stereoselective and Efficient Biocatalytic Synthesis of Chiral Syn-Aryl β-Hydroxy α-Amino Esters
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
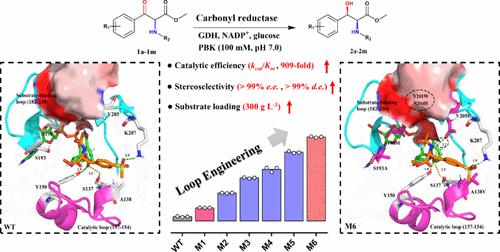
Chiral aryl β-hydroxy α-amino acids and their derivatives are essential pharmaceutical intermediates, widely used as chiral building blocks in drug synthesis. Nevertheless, the task of creating effective, extremely selective, and ecofriendly catalytic techniques for their production continues to be a major obstacle. Herein, loop engineering, combinatorial active-site saturation test/iterative saturation mutation (CAST/ISM), and the FuncLib strategies were applied to a carbonyl reductase (EaSDR6) from Exiguobacterium algae for the asymmetric biosynthesis of chiral syn-aryl β-hydroxy α-amino esters via dynamic reductive kinetic resolution (DYRKR) of aryl-α-amino β-keto esters with high yield and selectivity. Mutant M6 (A138 V/A190M/S193A/Y201W/N204A/V205E) exhibited a 909-fold improvement in catalytic efficiency (kcat/Km) and a significant increase in diastereoselectivity (from 59 to >99% d.e.) toward substrate 1a. It achieved 99% conversion within 8 h at a substrate concentration of 300 g L–1 (highest substrate loading reported), enabling gram-scale biosynthesis of the florfenicol precursor (2S,3R)-2a. Additionally, the VAF (A90 V/S193A/Y201F) mutant demonstrated high stereoselectivity (>94% e.e., 94–99% d.e.), desired conversions (>63%), and isolated yields (40–88%) for synthesizing diverse (2S,3R)-aryl β-hydroxy α-amino esters. Molecular dynamics simulations and quantum mechanical calculations unveiled that mutations induced a higher ratio of closed conformational states of the substrate-binding loop that shrinks cavity A, facilitating accommodation of the acetamide group of substrate 1a in this region and enhancing substrate binding and controlling diastereoselectivity (especially A190 M and S193A). This work highlights the potential of engineered carbonyl reductase in highly stereoselective and efficiently synthesizing chiral syn-aryl β-hydroxy α-amino esters and establishes a solid foundation for future large-scale industrial applications.
高立体选择性和高效生物催化合成手性合成芳基 β-羟基 α-氨基酯
手性芳基 β-羟基 α-氨基酸及其衍生物是重要的医药中间体,在药物合成中被广泛用作手性构件。然而,为其生产创造有效、选择性极高且环保的催化技术仍是一大障碍。在此,我们将环路工程、组合活性位点饱和度测试/迭代饱和度突变(CAST/ISM)和 FuncLib 策略应用于藻类外分支杆菌的羰基还原酶(EaSDR6),通过动态还原动力学解析(DYRKR)芳基-α-氨基 β-酮酯,以高产率和高选择性不对称生物合成手性合成芳基β-羟基 α-氨基酯。突变体 M6(A138 V/A190M/S193A/Y201W/N204A/V205E)的催化效率(kcat/Km)提高了 909 倍,对底物 1a 的非对映选择性显著提高(从 59% 提高到 99%)。在底物浓度为 300 g L-1 时(已报道的最高底物负载量),它在 8 小时内实现了 99% 的转化率,从而实现了克级规模的氟苯尼考前体 (2S,3R)-2a 的生物合成。此外,VAF(A90 V/S193A/Y201F)突变体在合成多种(2S,3R)-芳基β-羟基α-氨基酯时表现出了很高的立体选择性(>94% e.e.,94-99% d.e.)、理想的转化率(>63%)和分离产率(40-88%)。分子动力学模拟和量子力学计算揭示,突变诱导底物结合环的封闭构象态比例增加,从而缩小了空腔 A,有利于底物 1a 的乙酰胺基团容纳在该区域,并增强底物结合和控制非对映选择性(尤其是 A190 M 和 S193A)。这项工作凸显了工程羰基还原酶在高度立体选择性和高效合成手性合成芳基β-羟基α-氨基酯方面的潜力,并为未来的大规模工业应用奠定了坚实的基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


