Host cell glycosylation selects for infection with CCR5- versus CXCR4-tropic HIV-1
IF 20.5
1区 生物学
Q1 MICROBIOLOGY
引用次数: 0
Abstract
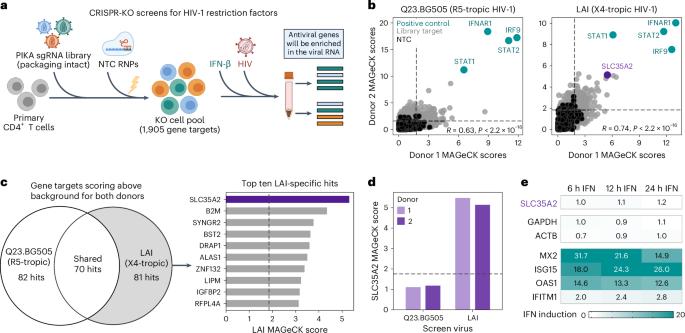
Human immunodeficiency virus type 1 (HIV-1) infection involves a selection bottleneck that leads to transmission of one or a few variants. C–C motif chemokine receptor 5 (CCR5) or C–X–C motif chemokine receptor 4 (CXCR4) can act as coreceptors for HIV-1 viral entry. However, initial infection mostly occurs via CCR5, despite abundant expression of CXCR4 on target cells. The host factors that influence HIV-1 susceptibility and selection during transmission are unclear. Here we conduct CRISPR–Cas9 screens and identify SLC35A2 (a transporter of UDP–galactose expressed in target cells in blood and mucosa) as a potent and specific CXCR4-tropic restriction factor in primary target CD4+ T cells. SLC35A2 inactivation, which resulted in truncated glycans, not only increased CXCR4-tropic infection levels but also decreased those of CCR5-tropic strains consistently. Single-cycle infections demonstrated that the effect is cell-intrinsic. These data support a role for a host protein that influences glycan structure in regulating HIV-1 infection. Host cell glycosylation may, therefore, affect HIV-1 selection during transmission in vivo. CRISPR–Cas9 screens in primary CD4+ T cells enable identification of SLC35A2 as a host factor that restricts CXCR4-tropic HIV-1 viral entry and promotes that of CCR5-tropic viruses.
宿主细胞糖基化选择感染 CCR5 型艾滋病毒-1 还是 CXCR4 型艾滋病毒-1
人类免疫缺陷病毒 1 型(HIV-1)感染涉及选择瓶颈,导致一种或几种变体的传播。C-C motif趋化因子受体 5(CCR5)或 C-X-C motif趋化因子受体 4(CXCR4)可作为 HIV-1 病毒进入的核心受体。然而,尽管靶细胞上大量表达 CXCR4,但最初的感染大多是通过 CCR5 发生的。在传播过程中,影响 HIV-1 易感性和选择的宿主因素尚不清楚。在这里,我们进行了 CRISPR-Cas9 筛选,发现 SLC35A2(一种在血液和粘膜靶细胞中表达的 UDP-半乳糖转运体)是主要靶 CD4+ T 细胞中一种有效且特异的 CXCR4-ropic 限制因子。SLC35A2 失活会产生截短的糖,不仅会增加 CXCR4-Tropic 感染水平,还会持续降低 CCR5-Tropic 菌株的感染水平。单循环感染表明,这种效应是细胞内在的。这些数据支持宿主蛋白质在调节 HIV-1 感染过程中发挥影响聚糖结构的作用。因此,宿主细胞糖基化可能会影响 HIV-1 在体内传播过程中的选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Microbiology
Immunology and Microbiology-Microbiology
CiteScore
44.40
自引率
1.10%
发文量
226
期刊介绍:
Nature Microbiology aims to cover a comprehensive range of topics related to microorganisms. This includes:
Evolution: The journal is interested in exploring the evolutionary aspects of microorganisms. This may include research on their genetic diversity, adaptation, and speciation over time.
Physiology and cell biology: Nature Microbiology seeks to understand the functions and characteristics of microorganisms at the cellular and physiological levels. This may involve studying their metabolism, growth patterns, and cellular processes.
Interactions: The journal focuses on the interactions microorganisms have with each other, as well as their interactions with hosts or the environment. This encompasses investigations into microbial communities, symbiotic relationships, and microbial responses to different environments.
Societal significance: Nature Microbiology recognizes the societal impact of microorganisms and welcomes studies that explore their practical applications. This may include research on microbial diseases, biotechnology, or environmental remediation.
In summary, Nature Microbiology is interested in research related to the evolution, physiology and cell biology of microorganisms, their interactions, and their societal relevance.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


