Amine headgroups in ionizable lipids drive immune responses to lipid nanoparticles by binding to the receptors TLR4 and CD1d
IF 26.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
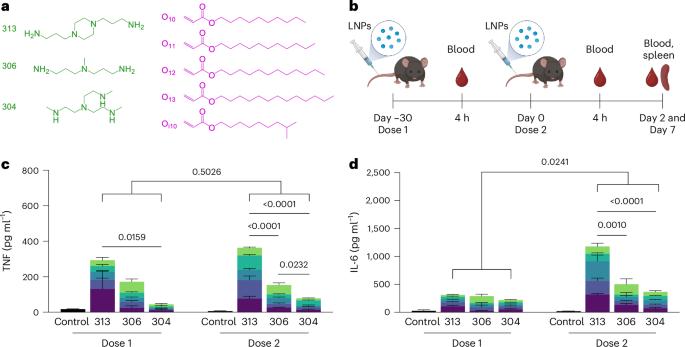
Lipid nanoparticles (LNPs) are the most clinically advanced delivery vehicle for RNA therapeutics, partly because of established lipid structure–activity relationships focused on formulation potency. Yet such knowledge has not extended to LNP immunogenicity. Here we show that the innate and adaptive immune responses elicited by LNPs are linked to their ionizable lipid chemistry. Specifically, we show that the amine headgroups in ionizable lipids drive LNP immunogenicity by binding to Toll-like receptor 4 and CD1d and by promoting lipid-raft formation. Immunogenic LNPs favour a type-1 T-helper-cell-biased immune response marked by increases in the immunoglobulins IgG2c and IgG1 and in the pro-inflammatory cytokines tumour necrosis factor, interferon γ and the interleukins IL-6 and IL-2. Notably, the inflammatory signals originating from these receptors inhibit the production of anti-poly(ethylene glycol) IgM antibodies, preventing the often-observed loss of efficacy in the LNP-mediated delivery of siRNA and mRNA. Moreover, we identified computational methods for the prediction of the structure-dependent innate and adaptive responses of LNPs. Our findings may help accelerate the discovery of well-tolerated ionizable lipids suitable for repeated dosing. Amine headgroups in ionizable lipids drive the immunogenicity of lipid nanoparticles by binding to Toll-like receptor 4 and CD1d and by promoting lipid-raft formation.
可离子化脂质中的胺头基通过与 TLR4 和 CD1d 受体结合驱动对纳米脂质颗粒的免疫反应
脂质纳米颗粒(LNPs)是临床上最先进的 RNA 治疗药物递送载体,部分原因是脂质结构与活性的关系已经确立,重点在于配方的有效性。然而,这些知识尚未扩展到 LNP 的免疫原性。在这里,我们证明了 LNPs 引起的先天性和适应性免疫反应与其可电离的脂质化学性质有关。具体来说,我们发现可离子化脂质中的胺头基通过与 Toll 样受体 4 和 CD1d 结合以及促进脂质移植物的形成来驱动 LNP 的免疫原性。免疫原性 LNP 有利于 1 型 T 辅助细胞为主的免疫反应,其特征是免疫球蛋白 IgG2c 和 IgG1 以及促炎细胞因子肿瘤坏死因子、干扰素 γ 和白细胞介素 IL-6 和 IL-2 的增加。值得注意的是,来自这些受体的炎症信号抑制了抗聚乙二醇 IgM 抗体的产生,从而避免了在 LNP 介导的 siRNA 和 mRNA 传输过程中经常出现的功效损失。此外,我们还找到了预测 LNPs 结构依赖性先天反应和适应性反应的计算方法。我们的发现可能有助于加快发现耐受性良好、适合重复给药的可电离脂质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Biomedical Engineering
Medicine-Medicine (miscellaneous)
CiteScore
45.30
自引率
1.10%
发文量
138
期刊介绍:
Nature Biomedical Engineering is an online-only monthly journal that was launched in January 2017. It aims to publish original research, reviews, and commentary focusing on applied biomedicine and health technology. The journal targets a diverse audience, including life scientists who are involved in developing experimental or computational systems and methods to enhance our understanding of human physiology. It also covers biomedical researchers and engineers who are engaged in designing or optimizing therapies, assays, devices, or procedures for diagnosing or treating diseases. Additionally, clinicians, who make use of research outputs to evaluate patient health or administer therapy in various clinical settings and healthcare contexts, are also part of the target audience.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


