Exome sequencing of 20,979 individuals with epilepsy reveals shared and distinct ultra-rare genetic risk across disorder subtypes
IF 21.2
1区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
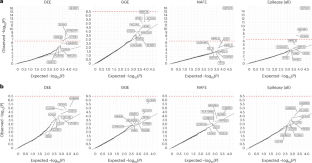
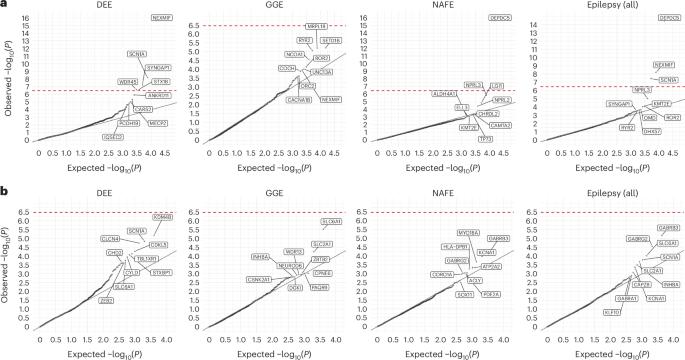
Identifying genetic risk factors for highly heterogeneous disorders such as epilepsy remains challenging. Here we present, to our knowledge, the largest whole-exome sequencing study of epilepsy to date, with more than 54,000 human exomes, comprising 20,979 deeply phenotyped patients from multiple genetic ancestry groups with diverse epilepsy subtypes and 33,444 controls, to investigate rare variants that confer disease risk. These analyses implicate seven individual genes, three gene sets and four copy number variants at exome-wide significance. Genes encoding ion channels show strong association with multiple epilepsy subtypes, including epileptic encephalopathies and generalized and focal epilepsies, whereas most other gene discoveries are subtype specific, highlighting distinct genetic contributions to different epilepsies. Combining results from rare single-nucleotide/short insertion and deletion variants, copy number variants and common variants, we offer an expanded view of the genetic architecture of epilepsy, with growing evidence of convergence among different genetic risk loci on the same genes. Top candidate genes are enriched for roles in synaptic transmission and neuronal excitability, particularly postnatally and in the neocortex. We also identify shared rare variant risk between epilepsy and other neurodevelopmental disorders. Our data can be accessed via an interactive browser, hopefully facilitating diagnostic efforts and accelerating the development of follow-up studies. In this largest whole-exome sequencing study of epilepsies to date, the Epi25 Collaborative identified extremely rare variants that confer risk for diverse epilepsy subtypes, highlighting roles in synaptic transmission and neuronal excitability.
对 20979 名癫痫患者的外显子组测序揭示了不同亚型癫痫的共同和独特的超罕见遗传风险
确定癫痫等高度异质性疾病的遗传风险因素仍然具有挑战性。据我们所知,迄今为止最大规模的癫痫全外显子组测序研究包含了超过 54,000 个人类外显子组,其中包括 20,979 名来自不同癫痫亚型的多基因祖先群体的深度表型患者和 33,444 名对照者,以研究赋予疾病风险的罕见变异。这些分析涉及七个单个基因、三个基因组和四个拷贝数变异,在整个外显子组中具有重要意义。编码离子通道的基因与多种癫痫亚型(包括癫痫性脑病、全身性癫痫和局灶性癫痫)有很强的相关性,而其他大多数基因的发现都具有亚型特异性,突显了基因对不同癫痫的不同贡献。结合罕见的单核苷酸/短插入和缺失变异、拷贝数变异和常见变异的结果,我们对癫痫的遗传结构有了更全面的认识,越来越多的证据表明同一基因上的不同遗传风险位点之间存在趋同性。热门候选基因在突触传递和神经元兴奋性中的作用非常丰富,尤其是在出生后和新皮层中。我们还发现了癫痫与其他神经发育障碍之间的共同罕见变异风险。我们的数据可以通过交互式浏览器访问,希望能为诊断工作提供便利,并加快后续研究的发展。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature neuroscience
医学-神经科学
CiteScore
38.60
自引率
1.20%
发文量
212
审稿时长
1 months
期刊介绍:
Nature Neuroscience, a multidisciplinary journal, publishes papers of the utmost quality and significance across all realms of neuroscience. The editors welcome contributions spanning molecular, cellular, systems, and cognitive neuroscience, along with psychophysics, computational modeling, and nervous system disorders. While no area is off-limits, studies offering fundamental insights into nervous system function receive priority.
The journal offers high visibility to both readers and authors, fostering interdisciplinary communication and accessibility to a broad audience. It maintains high standards of copy editing and production, rigorous peer review, rapid publication, and operates independently from academic societies and other vested interests.
In addition to primary research, Nature Neuroscience features news and views, reviews, editorials, commentaries, perspectives, book reviews, and correspondence, aiming to serve as the voice of the global neuroscience community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


