Elucidating the unexpected cell adhesive properties of agarose substrates. The effect of mechanics, fetal bovine serum and specific peptide sequences
IF 9.4
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
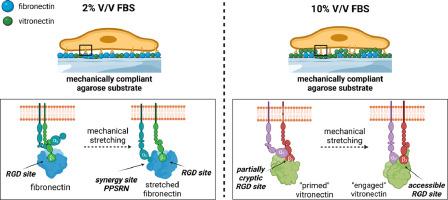
2D agarose substrates have recently been surprisingly shown to be permissive for cell adhesion, depending on their mechanics and the use of the adhesive proteins of fetal bovine serum (FBS) in the cell culture medium. Here, we elucidate how the cells exhibit two anchoring mechanisms depending on the amount of FBS. Under low FBS conditions, the cells recognize the surface-coupled adhesive sequences of fibronectin via the binding of the heterodimer α5β1 integrin. Functionality of the actomyosin axis and mechanoactivation of focal adhesion kinase (FAK) are essential for the stretching of the protein, thereby accessing the “synergy” PPSRN site and enhancing cell adhesion in combination with the downstream RGD motif. Under high FBS conditions, the specific peptide sequences are much less relevant as the adsorbed serum proteins conceal the coupled fibronectin and the cells recognize the adhesive protein vitronectin, which is constitutively present in FBS, via the binding of the heterodimer αvβ3 integrin. Similarly, the intracellular tension and FAK activity are decisive, which collectively indicate that the cells stretch the partially cryptic RGD site of vitronectin and thus make it more accessible for integrin binding. Both anchoring mechanisms only work properly if the agarose substrate is mechanically compliant in terms of linear stress-strain response, unraveling a critical balance between the mechanics of the agarose substrate and the presentation of the adhesive peptides.
Statement of significance
In the context of biomaterial design, agarose hydrogels are known to lack intrinsic cell-adhesive peptide motifs and are therefore commonly used for the development of non-permissive 2D substrates. However, we unexpectedly found that agarose hydrogels can become permissive substrates for cell adhesion, depending on a compliant mechanical response of the substrate and the use of fetal bovine serum (FBS) as protein reservoir in the cell culture medium. We describe here two anchoring mechanisms that cells harness to adhere to agarose substrates, depending on the amount of FBS. Our results will have a major impact on the field of mechanobiology and shed light on the central role of FBS as a natural source of adhesive proteins that could promote cell anchoring.
阐明琼脂糖基质意想不到的细胞粘附特性。力学、胎牛血清和特定肽序列的影响。
二维琼脂糖基底最近令人惊讶地显示出对细胞粘附的允许性,这取决于其力学和细胞培养基中胎牛血清(FBS)粘附蛋白的使用。在这里,我们阐明了细胞是如何根据 FBS 的含量表现出两种锚定机制的。在低 FBS 条件下,细胞通过结合异源二聚体 α5β1 整合素来识别纤维粘连蛋白的表面耦合粘附序列。肌动蛋白轴的功能性和病灶粘附激酶(FAK)的机械激活对蛋白质的伸展至关重要,从而进入 "协同 "PPSRN 位点,并与下游 RGD 矩阵结合增强细胞粘附性。在高 FBS 条件下,由于吸附的血清蛋白掩盖了耦合的纤连蛋白,细胞通过与异源二聚体 αvβ3 整合素的结合识别出 FBS 中构成性存在的粘附蛋白玻璃连蛋白,因此特异性肽序列的相关性大大降低。同样,细胞内张力和 FAK 活性也起着决定性作用,它们共同表明细胞拉伸了玻璃连蛋白部分隐蔽的 RGD 位点,从而使其更容易与整合素结合。这两种锚定机制只有在琼脂糖基底具有线性应力-应变反应的机械顺应性时才能正常工作,从而揭示了琼脂糖基底的机械性与粘附肽的呈现之间的关键平衡。意义说明:在生物材料设计方面,众所周知琼脂糖水凝胶缺乏内在的细胞粘附肽基团,因此常用于开发非粘附性二维基底。然而,我们意外地发现,琼脂糖水凝胶可以成为细胞粘附的容许基底,这取决于基底的顺应性机械响应以及细胞培养基中使用胎牛血清(FBS)作为蛋白质储备。我们在此描述细胞利用两种锚定机制粘附到琼脂糖基底上,这取决于 FBS 的用量。我们的研究结果将对机械生物学领域产生重大影响,并揭示了胎牛血清作为粘附蛋白天然来源的核心作用,可促进细胞锚定。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Acta Biomaterialia
工程技术-材料科学:生物材料
CiteScore
16.80
自引率
3.10%
发文量
776
审稿时长
30 days
期刊介绍:
Acta Biomaterialia is a monthly peer-reviewed scientific journal published by Elsevier. The journal was established in January 2005. The editor-in-chief is W.R. Wagner (University of Pittsburgh). The journal covers research in biomaterials science, including the interrelationship of biomaterial structure and function from macroscale to nanoscale. Topical coverage includes biomedical and biocompatible materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


