Eicosatrienoic acid enhances the quality of in vitro matured porcine oocytes by reducing PRKN-mediated ubiquitination of CISD2
IF 2.5
2区 农林科学
Q3 REPRODUCTIVE BIOLOGY
引用次数: 0
Abstract
Oocytes and early embryos are exposed to many uncontrollable factors that trigger endoplasmic reticulum (ER) stress during in vitro culture. Prevention of ER stress is an effective way to improve the oocyte maturation rate and oocyte quality. Increasing evidence suggests that dietary intake of sufficient n-3 polyunsaturated fatty acids (PUFAs) is associated with health benefits, particularly in the domain of female reproductive health. We found that supplementation of eicosatrienoic acid (ETA) during in vitro maturation (IVM) of oocyte significantly downregulated ER stress-related genes. Mitochondria-associated membranes (MAMs) are communications areas between the ER and mitochondria. Inositol 1,4,5-trisphosphate receptor (IP3R) is a key calcium channels in MAMs and, participates in the regulation of many cellular functions. Notably, the MAM area was significantly decreased in ETA-treated oocytes. CDGSH iron sulfur domain 2 (CISD2) is presents in MAMs, but its role in oocytes is unknown. ETA treatment significantly increased CISD2 expression, and siRNA-mediated knockdown of CISD2 blocked the inhibitory effect of ETA on IP3R. Transcriptomic sequencing and immunoprecipitation experiments showed that ETA treatment significantly decreased expression of the E3 ubiquitin ligase PRKN. PRKN induced ubiquitination and degradation of CISD2, indicating that the PRKN-mediated ubiquitin-proteasome system regulates CISD2. In conclusion, our study reveals the mechanism by which ETA supplementation during IVM alleviates mitochondrial calcium overload under ER stress conditions by decreasing PRKN-mediated ubiquitination of CISD2 and facilitating inhibition of IP3R by CISD2/BCL-2. This improves oocyte quality and subsequent embryo developmental competence prior to implantation.
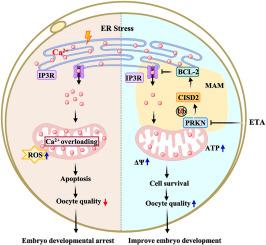
二十碳三烯酸可通过减少 PRKN 介导的 CISD2 泛素化来提高体外成熟猪卵母细胞的质量。
卵母细胞和早期胚胎在体外培养过程中会受到许多不可控因素的影响,从而引发内质网(ER)应激。预防内质网应激是提高卵母细胞成熟率和卵母细胞质量的有效方法。越来越多的证据表明,从膳食中摄入充足的 n-3 多不饱和脂肪酸(PUFAs)对健康有益,尤其是在女性生殖健康领域。我们发现,在卵母细胞体外成熟(IVM)过程中补充二十碳三烯酸(ETA)可显著下调ER应激相关基因。线粒体相关膜(MAM)是ER和线粒体之间的沟通区域。肌醇 1,4,5-三磷酸受体(IP3R)是 MAMs 中的一个关键钙通道,参与调控许多细胞功能。值得注意的是,经 ETA 处理的卵母细胞的 MAM 面积明显缩小。CDGSH 铁硫结构域 2(CISD2)存在于 MAMs 中,但其在卵母细胞中的作用尚不清楚。ETA 处理可明显增加 CISD2 的表达,而 siRNA 介导的 CISD2 敲除可阻断 ETA 对 IP3R 的抑制作用。转录组测序和免疫沉淀实验表明,ETA处理可明显降低E3泛素连接酶PRKN的表达。PRKN 诱导了 CISD2 的泛素化和降解,表明 PRKN 介导的泛素-蛋白酶体系统调控着 CISD2。总之,我们的研究揭示了在 IVM 期间补充 ETA,通过减少 PRKN 介导的 CISD2 泛素化,促进 CISD2/BCL-2 对 IP3R 的抑制,从而减轻 ER 应激条件下线粒体钙超载的机制。这就提高了卵母细胞的质量和随后胚胎植入前的发育能力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Theriogenology
农林科学-生殖生物学
CiteScore
5.50
自引率
14.30%
发文量
387
审稿时长
72 days
期刊介绍:
Theriogenology provides an international forum for researchers, clinicians, and industry professionals in animal reproductive biology. This acclaimed journal publishes articles on a wide range of topics in reproductive and developmental biology, of domestic mammal, avian, and aquatic species as well as wild species which are the object of veterinary care in research or conservation programs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


