A convenient strategy for mitigating microplastics in wastewater treatment using natural light and ZnO nanoparticles as photocatalysts: A mechanistic study
IF 3.5
3区 环境科学与生态学
Q2 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
Polypropylene microplastics (PPMPs) are one of the major emerging contaminants in the ecosystem due to their frequent usage and improper disposal practices. These PPMPs enter ecosystems via wastewater effluent plants and cause severe environmental health issues. In addition, quantifying PPMPs smaller than 50 μm in wastewater plant extraction is very difficult. Thus, the current study was designed to mitigate the PPMPs using zinc oxide nanoparticles (ZnONPs) as a photocatalyst under sunlight. The photocatalytic reaction was examined using spectroscopic techniques and microscopic imaging. The findings indicated that the weight loss percentage of PPMPs increased, and a decrease in UV–Vis DRS peak intensities was observed. The spectroscopic results elucidated the formation of free radicals, which affect the PPMPs and lead to the formation of carbonyl, allylic, and unsaturated groups. Further, EDS reports clarified that there is increased oxygen content due to the photooxidation process and the disintegration of the polymer chain owing to decreased carbon levels. Overall, ZnO photocatalyst absorbs photons from the visible spectrum of sunlight and forms free radicals, which affect the PPMPs to initiate polymer deterioration. Also, the current study revealed the mechanistic pathway of PPMP degradation under the photocatalytic reaction as proposed in the results obtained above.
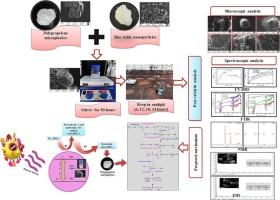
利用自然光和氧化锌纳米粒子作为光催化剂,在废水处理中减少微塑料的便捷策略:机理研究。
聚丙烯微塑料(PPMP)是生态系统中新出现的主要污染物之一,原因在于其频繁使用和不当的处置方式。这些 PPMP 通过污水处理厂进入生态系统,造成严重的环境健康问题。此外,对污水厂提取物中小于 50 μm 的 PPMP 进行量化非常困难。因此,本研究旨在利用氧化锌纳米颗粒(ZnONPs)作为光催化剂,在阳光下减轻 PPMPs 的影响。利用光谱技术和显微成像技术对光催化反应进行了检测。研究结果表明,PPMPs 的失重率增加,紫外可见 DRS 峰强度降低。光谱结果表明,自由基的形成影响了 PPMP,并导致羰基、烯丙基和不饱和基团的形成。此外,EDS 报告表明,光氧化过程导致氧含量增加,碳含量减少导致聚合物链解体。总之,氧化锌光催化剂吸收太阳光可见光谱中的光子并形成自由基,从而影响 PPMP,导致聚合物劣化。此外,本次研究还揭示了上述结果中提出的光催化反应下 PPMP 降解的机理途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of contaminant hydrology
环境科学-地球科学综合
CiteScore
6.80
自引率
2.80%
发文量
129
审稿时长
68 days
期刊介绍:
The Journal of Contaminant Hydrology is an international journal publishing scientific articles pertaining to the contamination of subsurface water resources. Emphasis is placed on investigations of the physical, chemical, and biological processes influencing the behavior and fate of organic and inorganic contaminants in the unsaturated (vadose) and saturated (groundwater) zones, as well as at groundwater-surface water interfaces. The ecological impacts of contaminants transported both from and to aquifers are of interest. Articles on contamination of surface water only, without a link to groundwater, are out of the scope. Broad latitude is allowed in identifying contaminants of interest, and include legacy and emerging pollutants, nutrients, nanoparticles, pathogenic microorganisms (e.g., bacteria, viruses, protozoa), microplastics, and various constituents associated with energy production (e.g., methane, carbon dioxide, hydrogen sulfide).
The journal''s scope embraces a wide range of topics including: experimental investigations of contaminant sorption, diffusion, transformation, volatilization and transport in the surface and subsurface; characterization of soil and aquifer properties only as they influence contaminant behavior; development and testing of mathematical models of contaminant behaviour; innovative techniques for restoration of contaminated sites; development of new tools or techniques for monitoring the extent of soil and groundwater contamination; transformation of contaminants in the hyporheic zone; effects of contaminants traversing the hyporheic zone on surface water and groundwater ecosystems; subsurface carbon sequestration and/or turnover; and migration of fluids associated with energy production into groundwater.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


