Discovery of organosulfur-based selective HDAC8 inhibitors with anti-neuroblastoma activity
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
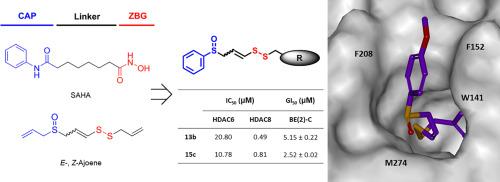
Histone deacetylases (HDACs) are important epigenetic regulators of gene expression and various cellular processes, and are potential targets for anticancer therapy. In particular, HDAC8 is a promising therapeutic target for childhood neuroblastoma. To date, five HDAC inhibitors have been approved as anticancer drugs; however, all are non-selective HDAC inhibitors with various side effects. Furthermore, many promising HDAC inhibitors incorporate hydroxamic acid as a zinc binding group (ZBG), which may be associated with toxicity. Therefore, identification of isoform-selective HDAC inhibitors with novel ZBG is crucial. Here, a series of sulfur-based selective HDAC8 inhibitors featuring a novel ZBG were identified by modifying the early hit, ajoene, a component of garlic. Structure-activity relationship studies uncovered potent and selective HDAC8 inhibitors, and docking studies provided a structural rationale for HDAC8 inhibitory activity. One of the potent compounds, (Z)-1-phenyl-7-(4-methoxyphenyl)-2,3,7-trithiahepta-4-ene-7-oxide (15c), exhibited antiproliferative activity, with a GI50 of 2 µM, against neuroblastoma cell lines. 15c also showed significant in vivo efficacy in a neuroblastoma BE(2)-C xenograft model.
发现具有抗神经母细胞瘤活性的有机硫基选择性 HDAC8 抑制剂。
组蛋白去乙酰化酶(HDAC)是基因表达和各种细胞过程的重要表观遗传调节因子,也是抗癌治疗的潜在靶点。其中,HDAC8 是治疗儿童神经母细胞瘤的一个很有前景的靶点。迄今为止,已有五种 HDAC 抑制剂被批准为抗癌药物,但它们都是具有各种副作用的非选择性 HDAC 抑制剂。此外,许多有前景的 HDAC 抑制剂都含有羟肟酸作为锌结合基团(ZBG),这可能与毒性有关。因此,鉴定具有新型 ZBG 的同工酶选择性 HDAC 抑制剂至关重要。在这里,通过对早期发现的大蒜成分琼脂进行修饰,发现了一系列以新型 ZBG 为特征的硫基选择性 HDAC8 抑制剂。结构-活性关系研究发现了强效和选择性 HDAC8 抑制剂,对接研究为 HDAC8 抑制活性提供了结构依据。其中一种强效化合物(Z)-1-苯基-7-(4-甲氧基苯基)-2,3,7-三硫杂庚烷-4-烯-7-氧化物(15c)对神经母细胞瘤细胞系具有抗增殖活性,其 GI50 为 2 µM。15c 还在神经母细胞瘤 BE(2)-C 异种移植模型中显示出显著的体内疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


