Development of intestinal colonic drug delivery systems for diverticular disease: A QbD approach
IF 4.3
3区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
Abstract
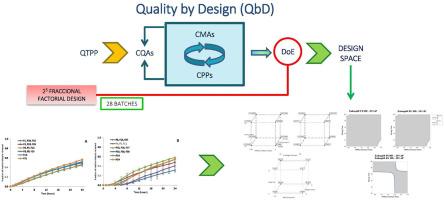
This study aimed to advance the development of intestinal colon-coated sustained-release matrix tablets of metronidazole for diverticulitis treatment, employing the Quality by Design (QbD) methodology. Comprehensive Risk analysis and Risk evaluation were conducted to assess the potential risks associated with Critical Material Attributes (CMA) and Critical Process Parameters (CPP). Ishikawa diagram, color-coded risk classification and the Risk Priority Number (RPN) were used as tools for risk evaluation. A Design of Experiments (DoE) was executed using a fractional factorial design, incorporating five key factors derived from the Risk analysis and Risk evaluation. Two levels and a central point were established for each factor, resulting in 28 batches of coated tablets. The manufacturing process involved direct compression, followed by a coating process using pH-dependent or time-dependent polymers. Characterization and dissolution studies were conducted on all batches, and the obtained results underwent analysis of variance (ANOVA).
The findings demonstrated the robustness and reproducibility of both the direct compression and coating processes. Statistical analysis identified HPMC/chitosan ratio, blending time, coating polymer, and coating weight gain as factors significantly impacting drug release. A Design Space was established to delineate the interplay of these factors, offering insights into various combinations influencing drug release behavior. Thus, the design space for 10 % weight gain formulations includes a range of HPMC/CH ratios between 2.7–3 and mixing times between 10 and 12 min; for 20 % weight gain formulations it includes a range of HPMC/CH ratios up to 2 and mixing times between 10 and 16 min. Multiple Linear Regression between technological and biopharmaceutical variables were optimized facilitating scale-up operations. Batches with a 10 % weight increase and varied HPMC viscosity grades and coating polymers achieve ∼50 % drug release at 24 h; however, batches with a 20 % weight increase along, with either high proportions of HPMC and short blending times or low proportions of HPMC and longer blending times, achieve slow release of metronidazole. This study contributes to optimizing metronidazole colonic delivery systems, enhancing their potential efficacy in diverticulitis treatment.
开发治疗憩室疾病的肠道结肠给药系统:一种 QbD 方法。
本研究旨在采用质量源于设计(QbD)方法,推进用于治疗憩室炎的甲硝唑肠道结肠包衣缓释基质片的开发。研究人员进行了全面的风险分析和风险评估,以评估与关键材料属性(CMA)和关键工艺参数(CPP)相关的潜在风险。石川图、彩色编码风险分类和风险优先序号 (RPN) 被用作风险评估的工具。实验设计(DoE)采用分数因子设计,纳入了从风险分析和风险评估中得出的五个关键因素。为每个因素确定了两个水平和一个中心点,最终确定了 28 个批次的包衣片剂。生产工艺包括直接压片,然后使用随 pH 值变化或随时间变化的聚合物进行包衣。对所有批次进行了表征和溶出研究,并对所得结果进行了方差分析(ANOVA)。研究结果表明,直接压缩和包衣工艺都具有稳健性和可重复性。统计分析表明,HPMC/壳聚糖比例、混合时间、包衣聚合物和包衣增重是影响药物释放的重要因素。建立了一个设计空间来描述这些因素之间的相互作用,以便深入了解影响药物释放行为的各种组合。因此,增重 10%配方的设计空间包括 2.7-3 之间的 HPMC/CH 比率和 10-12 分钟之间的混合时间;增重 20%配方的设计空间包括高达 2 的 HPMC/CH 比率和 10-16 分钟之间的混合时间。对技术和生物制药变量之间的多重线性回归进行了优化,以促进放大操作。重量增加 10%、HPMC 粘度等级和包衣聚合物不同的批次在 24 小时内药物释放量达到 50%;然而,重量增加 20%、HPMC 比例高、混合时间短或 HPMC 比例低、混合时间长的批次,甲硝唑释放缓慢。这项研究有助于优化甲硝唑结肠给药系统,提高其治疗憩室炎的潜在疗效。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
9.60
自引率
2.20%
发文量
248
审稿时长
50 days
期刊介绍:
The journal publishes research articles, review articles and scientific commentaries on all aspects of the pharmaceutical sciences with emphasis on conceptual novelty and scientific quality. The Editors welcome articles in this multidisciplinary field, with a focus on topics relevant for drug discovery and development.
More specifically, the Journal publishes reports on medicinal chemistry, pharmacology, drug absorption and metabolism, pharmacokinetics and pharmacodynamics, pharmaceutical and biomedical analysis, drug delivery (including gene delivery), drug targeting, pharmaceutical technology, pharmaceutical biotechnology and clinical drug evaluation. The journal will typically not give priority to manuscripts focusing primarily on organic synthesis, natural products, adaptation of analytical approaches, or discussions pertaining to drug policy making.
Scientific commentaries and review articles are generally by invitation only or by consent of the Editors. Proceedings of scientific meetings may be published as special issues or supplements to the Journal.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


