LYCHOS is a human hybrid of a plant-like PIN transporter and a GPCR
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
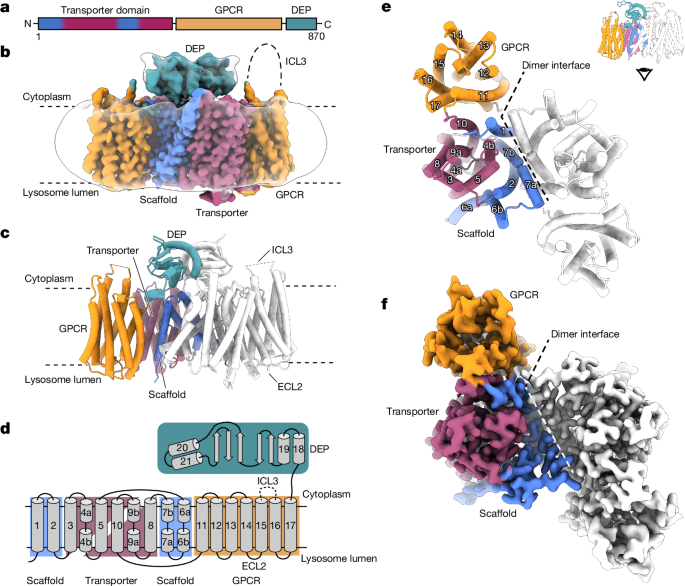
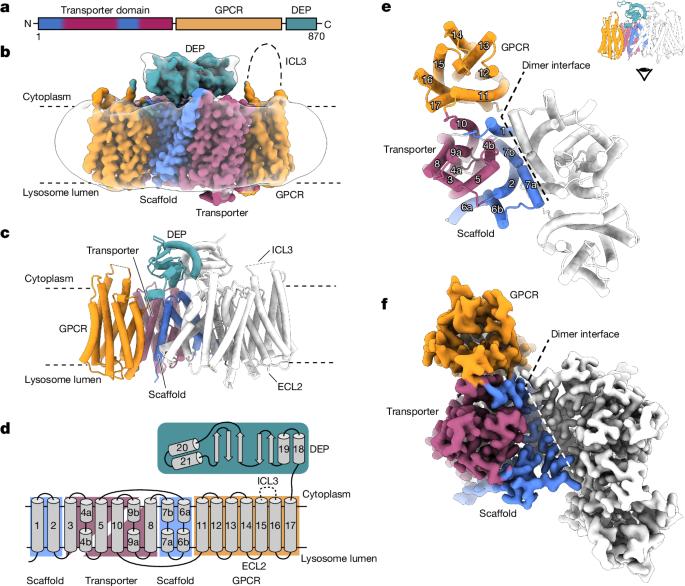
Lysosomes have crucial roles in regulating eukaryotic metabolism and cell growth by acting as signalling platforms to sense and respond to changes in nutrient and energy availability1. LYCHOS (GPR155) is a lysosomal transmembrane protein that functions as a cholesterol sensor, facilitating the cholesterol-dependent activation of the master protein kinase mechanistic target of rapamycin complex 1 (mTORC1)2. However, the structural basis of LYCHOS assembly and activity remains unclear. Here we determine several high-resolution cryo-electron microscopy structures of human LYCHOS, revealing a homodimeric transmembrane assembly of a transporter-like domain fused to a G-protein-coupled receptor (GPCR) domain. The class B2-like GPCR domain is captured in the apo state and packs against the surface of the transporter-like domain, providing an unusual example of a GPCR as a domain in a larger transmembrane assembly. Cholesterol sensing is mediated by a conserved cholesterol-binding motif, positioned between the GPCR and transporter domains. We reveal that the LYCHOS transporter-like domain is an orthologue of the plant PIN-FORMED (PIN) auxin transporter family, and has greater structural similarity to plant auxin transporters than to known human transporters. Activity assays support a model in which the LYCHOS transporter and GPCR domains coordinate to sense cholesterol and regulate mTORC1 activation. Cryo-electron microscopy structures of the human lysosomal transmembrane protein LYCHOS show that it comprises a transporter-like domain fused to a G-protein-coupled receptor, and that the transporter domain is similar to the plant PIN family.
LYCHOS 是一种类似植物 PIN 转运体和 GPCR 的人类混合体
溶酶体在调节真核生物新陈代谢和细胞生长方面起着至关重要的作用,它是感知和响应营养和能量供应变化的信号平台1。LYCHOS(GPR155)是一种溶酶体跨膜蛋白,具有胆固醇传感器的功能,可促进胆固醇依赖性激活雷帕霉素复合体 1(mTORC1)2 的主蛋白激酶机制靶标。 然而,LYCHOS 的组装和活性的结构基础仍不清楚。在这里,我们确定了人类 LYCHOS 的几种高分辨率冷冻电镜结构,揭示了一个类似于转运体的结构域与一个 G 蛋白偶联受体(GPCR)结构域融合的同源二聚体跨膜组装。B2类GPCR结构域在apo状态下被捕获,并靠在类转运体结构域的表面,提供了一个不同寻常的例子,说明在更大的跨膜组装中,GPCR是一个结构域。胆固醇感应是由位于 GPCR 和转运体结构域之间的保守的胆固醇结合基团介导的。我们发现,LYCHOS 的类转运体结构域是植物 PIN-FORMED (PIN)辅酶转运体家族的直系同源物,与已知的人类转运体相比,它与植物辅酶转运体的结构相似性更高。活性测定支持一种模型,即 LYCHOS 转运体和 GPCR 结构域协调感知胆固醇并调节 mTORC1 的激活。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


