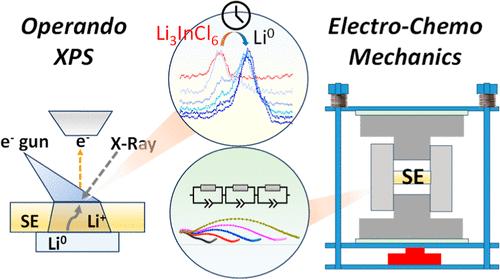
Evolution of Interfacial Electro-Chemo-Mechanics between Lithium Metal and Halide Solid Electrolyte
IF 7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The electro-chemo-mechanic phenomena that play a crucial role in the stability of halide solid electrolytes with Li metal at the interface are unknown. Moreover, in a halide SE, the central M atom is known to dictate its reactivity with Li metal. To understand this chemical composition-dependent reactivity of halide SE, we have taken three halide solid electrolytes, namely Li3InCl6, Li2ZrCl6, and Li3YCl6. Here, we use operando X-ray photoelectron spectroscopy during Li plating to understand the reaction kinetics leading to the interphase evolution and interphase composition. The interphase evolution in symmetric cells was further monitored by operando electrochemical impedance spectroscopy and operando pressure measurements, showing the complex intertwining of molar volume change, void formation, and interphase growth. The as-grown interphase was visualized by focused ion beam scanning electron microscopy. The chemical reactivity was confirmed by bond strength calculations using operando synchrotron X-ray diffraction. We confirm volume changes due to molar volume mismatch leading to different microstructures of interphases for the three-halide solid electrolytes, which are correlated to the impedance growth during electrochemistry by operando impedance measurements, showing molar volume change has a drastic effect on the reactivity.
金属锂与卤化物固体电解质之间的界面电化学力学演变
在卤化物固体电解质与锂金属的界面稳定性中起关键作用的电化学机械现象尚不清楚。此外,在卤化物 SE 中,已知中心 M 原子决定了其与锂金属的反应性。为了了解卤化物 SE 随化学成分变化的反应性,我们选择了三种卤化物固体电解质,即 Li3InCl6、Li2ZrCl6 和 Li3YCl6。在此,我们利用锂电镀过程中的操作性 X 射线光电子能谱来了解导致相间演化和相间组成的反应动力学。通过操作电化学阻抗光谱和操作压力测量进一步监测了对称电池中的相间演化,显示了摩尔体积变化、空隙形成和相间生长的复杂交织。聚焦离子束扫描电子显微镜可观察到生长的相间。利用操作同步辐射 X 射线衍射进行的键强度计算证实了化学反应性。我们证实了摩尔体积失配引起的体积变化,从而导致三卤化物固体电解质的相间微观结构不同,并通过操作阻抗测量将其与电化学过程中的阻抗增长联系起来,表明摩尔体积变化对反应性有很大影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chemistry of Materials
工程技术-材料科学:综合
CiteScore
14.10
自引率
5.80%
发文量
929
审稿时长
1.5 months
期刊介绍:
The journal Chemistry of Materials focuses on publishing original research at the intersection of materials science and chemistry. The studies published in the journal involve chemistry as a prominent component and explore topics such as the design, synthesis, characterization, processing, understanding, and application of functional or potentially functional materials. The journal covers various areas of interest, including inorganic and organic solid-state chemistry, nanomaterials, biomaterials, thin films and polymers, and composite/hybrid materials. The journal particularly seeks papers that highlight the creation or development of innovative materials with novel optical, electrical, magnetic, catalytic, or mechanical properties. It is essential that manuscripts on these topics have a primary focus on the chemistry of materials and represent a significant advancement compared to prior research. Before external reviews are sought, submitted manuscripts undergo a review process by a minimum of two editors to ensure their appropriateness for the journal and the presence of sufficient evidence of a significant advance that will be of broad interest to the materials chemistry community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


